Dinitrogen difluoride
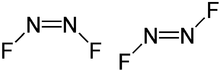 cis-Dinitrogen difluoride (left) and trans-dinitrogen difluoride (right) | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
cis- or trans-dinitrogen difluoride | |||
| Other names
cis- or trans-difluorodiazene | |||
| Identifiers | |||
| |||
3D model (JSmol) |
| ||
| ChemSpider |
| ||
| |||
| |||
| Identifiers | |||
| |||
3D model (JSmol) |
| ||
| ChemSpider |
| ||
| |||
| |||
| Properties | |||
| N2F2 | |||
| Molar mass | 66.010 g/mol | ||
| Appearance | colorless gas | ||
| Density | 2.698 g/L | ||
| Melting point | cis: < −195 °C (−319.0 °F; 78.1 K) trans: −172 °C | ||
| Boiling point | cis: −105.75 °C (−158.35 °F; 167.40 K) trans: −111.45 °C | ||
| cis: 0.16 D trans: 0 D | |||
| Thermochemistry | |||
Std enthalpy of formation (ΔfH |
cis: 69.5 kJ/mol trans: 82.0 kJ/mol | ||
| Related compounds | |||
Other cations |
azo compounds diazene | ||
Related Binary fluoro-azanes |
nitrogen trifluoride tetrafluorohydrazine | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Dinitrogen difluoride is a chemical compound with the formula N2F2. It is a gas at room temperature, and was first identified in 1952 as the thermal decomposition product of the azide N3F. It has the structure F−N=N−F and exists in both a cis- and trans-form.
Isomers
The cis configuration lies in a C2v symmetry and the trans-form has a symmetry of C2h. These isomers are thermally interconvertible but can be separated by low temperature fractionation. The trans-form is less thermodynamically stable but can be stored in glass vessels. The cis-form attacks glass over a time scale of about 2 weeks to form silicon tetrafluoride and nitrous oxide:[2]
- 2 N2F2 + SiO2 → SiF4 + 2 N2O
Preparation
Most preparations of dinitrogen difluoride give mixtures of the two isomers, but they can be prepared independently.
An aqueous method involves N,N-difluorourea with concentrated potassium hydroxide. This gives a 40% yield with three times more of the trans isomer.[3]
Difluoramine forms a solid unstable compound with potassium fluoride (or rubidium fluoride or caesium fluoride) which decomposes to dinitrogen difluoride.[3]
It can also be prepared by photolysis of tetrafluorohydrazine and bromine[4]:
Reactions
The cis form of dinitrogen difluoride will react with strong fluoride ion acceptors such as antimony pentafluoride to form the N2F+ cation.
- N2F2 + SbF5 → N2F+[SbF6]−
In the solid phase, the observed N=N and N−F bond distances in the N2F+ cation are 1.089(9) and 1.257(8) Å respectively, among the shortest experimentally observed N−N and N−F bonds.
References
- ↑ Lide, David R. (1998). Handbook of Chemistry and Physics (87th ed.). Boca Raton, FL: CRC Press. pp. 4–73, 5–15, 9–46. ISBN 0-8493-0594-2.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0-08-037941-9.
- 1 2 Sykes, A. G. (1989-07-17). Advances in Inorganic Chemistry. Academic Press. p. 171. ISBN 9780080578828. Retrieved 21 June 2014.
- ↑ Leon M. Zaborowski et al. (1973), Aaron Wold and John K. Ruff, ed., "Chlorodifluoroamine and Difluorodiazene - B. Difluorodiazene (Dinitrogen difluoride)" (in German), Inorganic Syntheses (McGraw-Hill Book Company, Inc.) 14: pp. 34–39
-Dinitrogen-difluoride-3D-balls.png)
-Dinitrogen-difluoride-3D-balls.png)