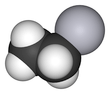
Ethylmercury
| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| |||
| |||
| Properties | |||
| C2H5Hg+ | |||
| Molar mass | 229.65 g/mol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Ethylmercury (sometimes ethyl mercury) is a cation composed of an organic CH3CH2- species (an ethyl group) bound to a mercury(II) centre, making it a type of organometallic cation, and giving it a chemical formula is C2H5Hg+. It is one of the metabolites of thiomersal (thimerosal or sodium ethyl mercuric thiosalicylate), which is used as a preservative in some vaccines.[1] The term "ethylmercury" is also sometimes used as a generic term to describe organomercury compounds which include the ethylmercury functional group, such as ethylmercury chloride and ethylmercury urea.
Chemistry
Given the comparable electronegativities of mercury and carbon, the mercury-carbon bond is best described as covalent.[2]:p. 79 In its parent and related compounds (e.g., thimerosal), the bond angle of RHgX species are linear (due to sp or dz2s hybridization of Hg).[2]:p. 79
The route to the toxicity of inorganic mercury lies through its biological methylation, and the extreme toxicity of the methylmercury cation, CH3Hg+, the relevance to ethylmercury, CH3CH2Hg+ having yet to be established, but with clear evidence for conversion of ethylmercury to inorganic mercury (see below).[2]:p. 82ff
Toxicity
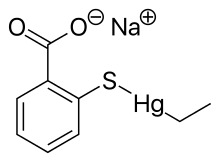
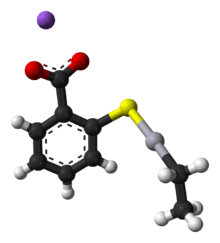
The toxicity of ethylmercury, for instance as it derives in vivo from thimerosal, is not well studied, and for many years, studies of methylmercury were used as a basis to predict the safety and estimate the risk of thimerosal use. Methylmercury and ethylmercury distribute to all body tissues, crossing the blood–brain barrier and the placental barrier, and ethylmercury also moves freely throughout the body.[3] Risk assessment for effects on the human nervous system have been made by extrapolating from dose-response relationships for methylmercury.[4] Clifton has offered the estimate that ethylmercury clears from blood with a half-life of seven to 10 days in adult humans.[5]
However, preliminary direct evidence from a 2005 animal study, subsequently summarised in an NIAID fact sheet on the use of thimerosal, suggested that methylmercury is an inadequate reference compound for evaluating the toxicology of ethylmercury, because the two compounds differ significantly in the ratio of organic to inorganic mercury each produces in the brain, as well as in their individual tissue distributions and clearance rates.[6][7][8][9] Taken together, the researchers conclude from their monkey study of ADME for inoculated thimerosal-derived ethylmercury and the stomach-administered methylmercury that past and ongoing studies of methylmercury are unsuitable as a basis for evaluating thimerosal toxicity, and that thimerosal risk assessment "based on blood mercury measurements may not be valid" [emphasis added].[10]
Public health concerns
Concerns based on extrapolations from methylmercury caused thiomersal to be removed from U.S. childhood vaccines, starting in 1999. Clarkson has argued that risk assessments based on methylmercury were overly conservative, in light of observations that ethylmercury is eliminated from the body and the brain significantly faster than methylmercury. Moreover, Clarkson has argued that inorganic mercury metabolized from ethylmercury, despite its much longer half-life in the brain, is much less toxic than the inorganic mercury produced from mercury vapor, for reasons not yet understood.[4]
Environmental accumulation
Unlike methylmercury, ethylmercury has not been found to bioaccumulate.
See also
References and notes
- ↑ "Thimerosal in vaccines". Center for Biologics Evaluation and Research, U.S. Food and Drug Administration. 2008-06-03. Retrieved 2008-07-25.
- 1 2 3 Elschenbroich C (2016). "Main-Group Organometallics [§6.2.3 Organomercury Compounds]". Organometallics (3rd ed.). New York, NY: John Wiley & Sons. pp. 78–86. ISBN 978-3-527-80514-3. Retrieved 13 February 2017.
- ↑ Clarkson TW, Vyas JB, Ballatori N (October 2007). "Mechanisms of mercury disposition in the body". American Journal of Industrial Medicine. 50 (10): 757–64. doi:10.1002/ajim.20476. PMID 17477364.
- 1 2 Clarkson TW, Magos L (September 2006). "The toxicology of mercury and its chemical compounds". Critical Reviews in Toxicology. 36 (8): 609–62. doi:10.1080/10408440600845619. PMID 16973445.
- ↑ Clifton JC (April 2007). "Mercury exposure and public health". Pediatric Clinics of North America. 54 (2): 237–69, viii. doi:10.1016/j.pcl.2007.02.005. PMID 17448359.
- ↑ Mitchell WJ (2005). "Carbohydrate assimilation by saccharolytic clostridia". Research in Microbiology. 143 (3): 245–50. doi:10.1289/ehp.113-a543. PMC 1280369.
- ↑ Burbacher TM, Shen DD, Liberato N, Grant KS, Cernichiari E, Clarkson T (August 2005). "Comparison of blood and brain mercury levels in infant monkeys exposed to methylmercury or vaccines containing thimerosal". Environmental Health Perspectives. 113 (8): 1015–21. doi:10.1289/ehp.7712. PMC 1280342. PMID 16079072.
- ↑ NIAID Research on Thimerosal Archived April 27, 2006, at the Wayback Machine.
- ↑ The study followed these pharmacokinetic parameters in 17 newborn monkeys exposed to ethylmercury via thimerosal in a pattern to mimic childhood vaccination versus 17 that received comparable doses per kilogram body weight of methylmercury by stomach tube (and 7 unexposed control animals), researchers observed:
- comparable tissue distributions and initial absorption rates for both compounds (i.e., for both treatment groups);
- total mercury clearance being more rapid for the thimerosal/ethylmercury treatment group relative to the methylmercury group (half-life 24.2 days, vs. 59.5 days), with rapid decline in total blood mercury between doses for thimerosal/ethyl- (half life 6,9 days) versus an accumulating value for the methylmercury (half-life 19.1 days)—with estimated clearance rate for the thimerosal-derived mercury being >5-fold higher—and with detectable levels after final dose for the methylmercury remaining after almost a month; and
- that while total mercury values measured in subject brains were ca. 3–4 times lower in the thimerosal treatment group than in the methylmercury treatment group, measures of inorganic mercury concentrations in brain were ca. twice as high in the thimerosal treatment group, and the "proportion of inorganic mercury in the brain was much higher in the thimerosal group (21–86% of total mercury) compared to the methylmercury group (6–10%)".
- ↑ As of the time of the report (2005), the estimated half-life of inorganic mercury in the brain was known to be much longer than organic mercury—with the estimated value of the inorganic being more than a year—but the potential risk posed by the presence of inorganic mercury in the developing brain was uncharacterized. Hence, further research on the ADME of thimerosal and of its neurotoxicity was called for by the report, in light of research linking "persistent inorganic mercury exposure" with neurological effects (and possible relevance to autism in children). See Barrett (2005) and Burbacher, et al. (2005), op. cit.
Further reading
- Mitchell WJ (2005). "Carbohydrate assimilation by saccharolytic clostridia". Research in Microbiology. 143 (3): 245–50. doi:10.1289/ehp.113-a543. PMC 1280369.
- DHHS ATSDR, US (March 2013). "Addendum to the Toxicological Profile for Mercury (Alkyl and Dialkyl Compounds)" (PDF). CDC.gov. Retrieved 13 February 2017.
- EPA, OA, US. "Thimerosal in Vaccines". EPA.gov. Retrieved 13 February 2017.
- Santos JC, da Silva IM, Braga TC, de Fátima Â, Figueiredo IM, Santos JC (February 2018). "Thimerosal changes protein conformation and increase the rate of fibrillation in physiological conditions: Spectroscopic studies using bovine serum albumin (BSA)". International Journal of Biological Macromolecules. 113: 1032–1040. doi:10.1016/j.ijbiomac.2018.02.116. PMID 29476861.