Electrochromism
Electrochromism is the phenomenon displayed by some materials of reversibly changing colour stimulated by redox reactions. Various types of materials and structures can be used to construct electrochromic devices. Electrochromic displays are based on any material that changes color depending on the applied potential.[1]
Principles
As the color change is persistent and energy need only be applied to affect a change, electrochromic materials are used to control the amount of light and heat allowed to pass through windows ("smart windows"), One popular application is in the automobile industry where it is used to automatically tint rear-view mirrors in various lighting conditions.
Electrochromic materials
Among the metal oxides, tungsten oxide (WO3), has been the most extensively studied material.
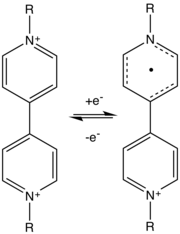
For organic materials, viologens have been commercialized on small scale. A variety of conducting polymers are also of interest, including polypyrrole, PEDOT, and polyaniline. Viologen is used in conjunction with titanium dioxide (TiO2) in the creation of small digital displays. It is hoped that these displays will replace liquid crystal displays as the viologen, which is typically dark blue, has a high contrast compared to the bright white of the titania, thereby providing the display high visibility.
Applications
.jpg)
Used in the production of electrochromic windows or smart glass and more recently electrochromic displays on paper substrate as anti-counterfeiting systems integrated on packaging. NiO materials have been widely studied as counter electrodes for complementary electrochromic devices, in particular, smart windows.
ICE 3 high speed trains use electrochromatic glass panels between the passenger compartment and the driver's cabin. The standard mode is clear, and can be switched by the driver to frosted/translucent, mainly to conceal "unwanted sights" from passengers' view, for example in the case of (human) obstacles. Electrochromic windows are used in the Boeing 787 Dreamliner.
See also
Further reading
- Granqvist, C.G. (2002) [1995]. Handbook of Inorganic Electrochromic Materials. Elsevier. ISBN 978-0-08-053290-5.
- Lin, Feng; Nordlund, Dennis; Weng, Tsu-Chien; et al. (2013). "Origin of Electrochromism in High-Performing Nanocomposite Nickel Oxide". ACS Applied Materials & Interfaces. American Chemical Society. 5 (9): 3643–3649. doi:10.1021/am400105y.
- Moulki,, Hakim; Park, Dae Hoon; Min, Bong-Ki; et al. (15 July 2012). "Improved electrochromic performances of NiO based thin films by lithium addition: From single layers to devices". Electrochimica Acta. 74: 46–52. doi:10.1016/j.electacta.2012.03.123.
- Lin, Feng; Cheng, Jifang; Engtrakul, Chaiwat; et al. (2012). "In situ crystallization of high performing WO3-based electrochromic materials and the importance for durability and switching kinetics". Journal of Materials Chemistry. 22: 16817–16823. doi:10.1039/c2jm32742b.
- Deb, S. K. (1969). "A Novel Electrophotographic System". Applied Optics. 8 (S1): 192–195. Bibcode:1969ApOpt...8S.192D. doi:10.1364/AO.8.S1.000192.
- Deb, S. K. (1973). "Optical and photoelectric properties and colour centres in thin films of tungsten oxide". Philosophical Magazine. 27 (4): 801–822. Bibcode:1973PMag...27..801D. doi:10.1080/14786437308227562.
- Gillaspie, Dane T.; Tenent, Robert C.; Dillon, Anne C. (2010). "Metal-oxide films for electrochromic applications: present technology and future directions". Journal of Materials Chemistry. 20 (43): 9585–9592. doi:10.1039/C0JM00604A.
- Danine, A.; Cojocaru, L.; Faure, C.; et al. (20 May 2014). "Room Temperature UV treated WO3 thin films for electrochromic devices on paper substrate". Electrochimica Acta. 129: 113–119. doi:10.1016/j.electacta.2014.02.028.
- WO patent 2014135804, Danine, Abdelaadim; Faure, Cyril & Campet, Guy et al., "Electrochromic device comprising three or four layers", issued September 12, 2014
References
- 1 2 Mortimer, R. J. (2011). "Electrochromic Materials". Annu. Rev. Mater. Res. 41. pp. 241–268. Bibcode:2011AnRMS..41..241M. doi:10.1146/annurev-matsci-062910-100344.