EPB41L1
Band 4.1-like protein 1 is a protein that in humans is encoded by the EPB41L1 gene.[5][6][7]
Function
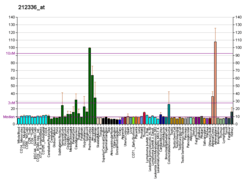
Erythrocyte membrane protein band 4.1 (EPB41) is a multifunctional protein that mediates interactions between the erythrocyte cytoskeleton and the overlying plasma membrane. The protein encoded by this gene is a neuronally-enriched protein that is structurally similar to EPB41. The encoded protein binds and stabilizes D2 and D3 dopamine receptors at the neuronal plasma membrane. Multiple transcript variants encoding different isoforms have been found for this gene, but the full-length nature of only two of them has been determined.[7]
Interactions
EPB41L1 has been shown to interact with:
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000088367 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000027624 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Kim AC, Van Huffel C, Lutchman M, Chishti AH (June 1998). "Radiation hybrid mapping of EPB41L1, a novel protein 4.1 homologue, to human chromosome 20q11.2-q12". Genomics. 49 (1): 165–6. doi:10.1006/geno.1998.5212. PMID 9570967.
- ↑ Peters LL, Weier HU, Walensky LD, Snyder SH, Parra M, Mohandas N, Conboy JG (January 1999). "Four paralogous protein 4.1 genes map to distinct chromosomes in mouse and human". Genomics. 54 (2): 348–50. doi:10.1006/geno.1998.5537. PMID 9828140.
- 1 2 "Entrez Gene: EPB41L1 erythrocyte membrane protein band 4.1-like 1".
- ↑ Ye K, Hurt KJ, Wu FY, Fang M, Luo HR, Hong JJ, Blackshaw S, Ferris CD, Snyder SH (December 2000). "Pike. A nuclear gtpase that enhances PI3kinase activity and is regulated by protein 4.1N". Cell. 103 (6): 919–30. doi:10.1016/S0092-8674(00)00195-1. PMID 11136977.
- 1 2 Binda AV, Kabbani N, Lin R, Levenson R (September 2002). "D2 and D3 dopamine receptor cell surface localization mediated by interaction with protein 4.1N". Mol. Pharmacol. 62 (3): 507–13. doi:10.1124/mol.62.3.507. PMID 12181426.
- ↑ Maximov A, Tang TS, Bezprozvanny I (February 2003). "Association of the type 1 inositol (1,4,5)-trisphosphate receptor with 4.1N protein in neurons". Mol. Cell. Neurosci. 22 (2): 271–83. doi:10.1016/s1044-7431(02)00027-1. PMID 12676536.
- ↑ Ye K, Compton DA, Lai MM, Walensky LD, Snyder SH (December 1999). "Protein 4.1N binding to nuclear mitotic apparatus protein in PC12 cells mediates the antiproliferative actions of nerve growth factor". J. Neurosci. 19 (24): 10747–56. PMID 10594058.
Further reading
- Calinisan V, Gravem D, Chen RP, Brittin S, Mohandas N, Lecomte MC, Gascard P (2006). "New insights into potential functions for the protein 4.1 superfamily of proteins in kidney epithelium". Front. Biosci. 11: 1646–66. doi:10.2741/1911. PMID 16368544.
- Nagase T, Ishikawa K, Nakajima D, Ohira M, Seki N, Miyajima N, Tanaka A, Kotani H, Nomura N, Ohara O (1997). "Prediction of the coding sequences of unidentified human genes. VII. The complete sequences of 100 new cDNA clones from brain which can code for large proteins in vitro". DNA Res. 4 (2): 141–50. doi:10.1093/dnares/4.2.141. PMID 9205841.
- Walensky LD, Blackshaw S, Liao D, Watkins CC, Weier HU, Parra M, Huganir RL, Conboy JG, Mohandas N, Snyder SH (1999). "A novel neuron-enriched homolog of the erythrocyte membrane cytoskeletal protein 4.1". J. Neurosci. 19 (15): 6457–67. PMID 10414974.
- Ye K, Compton DA, Lai MM, Walensky LD, Snyder SH (2000). "Protein 4.1N binding to nuclear mitotic apparatus protein in PC12 cells mediates the antiproliferative actions of nerve growth factor". J. Neurosci. 19 (24): 10747–56. PMID 10594058.
- Shen L, Liang F, Walensky LD, Huganir RL (2001). "Regulation of AMPA receptor GluR1 subunit surface expression by a 4. 1N-linked actin cytoskeletal association". J. Neurosci. 20 (21): 7932–40. PMID 11050113.
- Ye K, Hurt KJ, Wu FY, Fang M, Luo HR, Hong JJ, Blackshaw S, Ferris CD, Snyder SH (2001). "Pike. A nuclear gtpase that enhances PI3kinase activity and is regulated by protein 4.1N". Cell. 103 (6): 919–30. doi:10.1016/S0092-8674(00)00195-1. PMID 11136977.
- Binda AV, Kabbani N, Lin R, Levenson R (2002). "D2 and D3 dopamine receptor cell surface localization mediated by interaction with protein 4.1N". Mol. Pharmacol. 62 (3): 507–13. doi:10.1124/mol.62.3.507. PMID 12181426.
- Zhang S, Mizutani A, Hisatsune C, Higo T, Bannai H, Nakayama T, Hattori M, Mikoshiba K (2003). "Protein 4.1N is required for translocation of inositol 1,4,5-trisphosphate receptor type 1 to the basolateral membrane domain in polarized Madin-Darby canine kidney cells". J. Biol. Chem. 278 (6): 4048–56. doi:10.1074/jbc.M209960200. PMC 2366074. PMID 12444087.
- Coleman SK, Cai C, Mottershead DG, Haapalahti JP, Keinänen K (2003). "Surface expression of GluR-D AMPA receptor is dependent on an interaction between its C-terminal domain and a 4.1 protein". J. Neurosci. 23 (3): 798–806. PMID 12574408.
- Maximov A, Tang TS, Bezprozvanny I (2003). "Association of the type 1 inositol (1,4,5)-trisphosphate receptor with 4.1N protein in neurons". Mol. Cell. Neurosci. 22 (2): 271–83. doi:10.1016/S1044-7431(02)00027-1. PMID 12676536.
- Nagaraja GM, Kandpal RP (2004). "Chromosome 13q12 encoded Rho GTPase activating protein suppresses growth of breast carcinoma cells, and yeast two-hybrid screen shows its interaction with several proteins". Biochem. Biophys. Res. Commun. 313 (3): 654–65. doi:10.1016/j.bbrc.2003.12.001. PMID 14697242.
- Ballif BA, Villén J, Beausoleil SA, Schwartz D, Gygi SP (2005). "Phosphoproteomic analysis of the developing mouse brain". Mol. Cell. Proteomics. 3 (11): 1093–101. doi:10.1074/mcp.M400085-MCP200. PMID 15345747.
- Beausoleil SA, Villén J, Gerber SA, Rush J, Gygi SP (2006). "A probability-based approach for high-throughput protein phosphorylation analysis and site localization". Nat. Biotechnol. 24 (10): 1285–92. doi:10.1038/nbt1240. PMID 16964243.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.