Drosomycin
The drosomycin is antifungal peptide from Drosophila melanogaster and it is the first antifungal peptide isolated from insects. This peptide was discovered in 1994 by Fehlbaum and his team.[1] Expression of this peptide is inducible and in systemic way is regulated by Toll pathway,[2] while expression in respiratory tract is controlled by immune deficiency pathway (IMD).[3] It means that drosomycin, with other antimicrobial peptides (AMP) such as cecropins,[4][5] diptericin,[6] drosocin,[7] metchnikowin[8] and attacin[9] serves as a first line defense in case of septic wounds. However drosomycin is not expressed only in case of wound, but is also expressed constitutively in some parts of Drosophila´s body in all developmental stages (larvae, pupae, adult).[10]
Structure
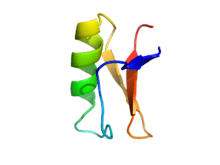
This peptide composed from 44 amino acids residues and has 4 disulphide bridges, which stabilized one α-helix and three stranded β-sheet. Due to this 4 bridges drosomycin has quite large resistance to proteases.[11][12][13] In drosomycin is appearing evolutionary conservative cysteine stabilized αβ motif, which can be also found in other Drosophila defensins or in some plant defensins. It is interesting, that drosomycin has bigger sequence similarity with these plant defensis, for example defensin from Raphanus sativus (about 40%), than with other insect defensins.[14] The structure was discovered in 1997 by Landon and his colleagues[15]
Drosomycin multigene family
From genetic point of view drosomycin is coded by 387 bp long gene Drs which lies on the third chromosome[16] clustered with 6 other genes (Dro1-6) and creating together drosomycin multigene family. However these genes don't have similar activities as it could look. Which has been experimentally proven by Yang and his colleagues.[17][18] Nevertheless, it's not as simple as it looks. In 2015 Gao and Zhu have found that in some Drosophila species (D. takahashii) some of these genes have been duplicated and this Drosophila has 11 genes in drosomycin multigene family in total.[19]
Function
It seems that drosomycin has about three major functions on fungi, the first is partial lysis of hyphae, second is inhibition of spore germination (in higher concentrations of drosomycin) and the last is delaying of hyphea growth, which leads to hyphae branching (in lower concentrations of drosomycin).[20] The exact mechanism of function to fungi still has to be clarified.
References
- ↑ Fehlbaum, P.; Bulet, P.; Michaut, L.; Lagueux, M.; Broekaert, W. F.; Hetru, C.; Hoffmann, J. A. (1994-12-30). "Insect immunity. Septic injury of Drosophila induces the synthesis of a potent antifungal peptide with sequence homology to plant antifungal peptides". Journal of Biological Chemistry. 269 (52): 33159–33163. ISSN 0021-9258. PMID 7806546.
- ↑ Lemaitre, Bruno; Nicolas, Emmanuelle; Michaut, Lydia; Reichhart, Jean-Marc; Hoffmann, Jules A (1996-09-20). "The Dorsoventral Regulatory Gene Cassette spätzle/Toll/cactus Controls the Potent Antifungal Response in Drosophila Adults". Cell. 86 (6): 973–983. doi:10.1016/S0092-8674(00)80172-5.
- ↑ Zhang, Z-T.; Zhu, S-Y. (2009-10-01). "Drosomycin, an essential component of antifungal defence in Drosophila". Insect Molecular Biology. 18 (5): 549–556. doi:10.1111/j.1365-2583.2009.00907.x. ISSN 1365-2583.
- ↑ Kylsten, P.; Samakovlis, C.; Hultmark, D. (1990-01-01). "The cecropin locus in Drosophila; a compact gene cluster involved in the response to infection". The EMBO Journal. 9 (1): 217–224. ISSN 0261-4189. PMC 551649. PMID 2104802.
- ↑ Tryselius, Y.; Samakovlis, C.; Kimbrell, D. A.; Hultmark, D. (1992-02-15). "CecC, a cecropin gene expressed during metamorphosis in Drosophila pupae". European Journal of Biochemistry. 204 (1): 395–399. ISSN 0014-2956. PMID 1740152.
- ↑ Wicker, C.; Reichhart, J. M.; Hoffmann, D.; Hultmark, D.; Samakovlis, C.; Hoffmann, J. A. (1990-12-25). "Insect immunity. Characterization of a Drosophila cDNA encoding a novel member of the diptericin family of immune peptides". Journal of Biological Chemistry. 265 (36): 22493–22498. ISSN 0021-9258. PMID 2125051.
- ↑ Bulet, P.; Dimarcq, J. L.; Hetru, C.; Lagueux, M.; Charlet, M.; Hegy, G.; Dorsselaer, A. Van; Hoffmann, J. A. (1993-07-15). "A novel inducible antibacterial peptide of Drosophila carries an O-glycosylated substitution". Journal of Biological Chemistry. 268 (20): 14893–14897. ISSN 0021-9258. PMID 8325867.
- ↑ Levashina, E. A.; Ohresser, S.; Bulet, P.; Reichhart, J. M.; Hetru, C.; Hoffmann, J. A. (1995-10-15). "Metchnikowin, a novel immune-inducible proline-rich peptide from Drosophila with antibacterial and antifungal properties". European Journal of Biochemistry. 233 (2): 694–700. ISSN 0014-2956. PMID 7588819.
- ↑ Asling, B.; Dushay, M. S.; Hultmark, D. (1995-04-01). "Identification of early genes in the Drosophila immune response by PCR-based differential display: the Attacin A gene and the evolution of attacin-like proteins". Insect Biochemistry and Molecular Biology. 25 (4): 511–518. ISSN 0965-1748. PMID 7742836.
- ↑ Ferrandon, D.; Jung, A. C.; Criqui, M.; Lemaitre, B.; Uttenweiler-Joseph, S.; Michaut, L.; Reichhart, J.; Hoffmann, J. A. (1998-08-10). "A drosomycin-GFP reporter transgene reveals a local immune response in Drosophila that is not dependent on the Toll pathway". The EMBO Journal. 17 (5): 1217–1227. doi:10.1093/emboj/17.5.1217. ISSN 0261-4189. PMC 1170470. PMID 9482719.
- ↑ P. Fehlbaum, P. Bulet, L. Michaut, M. Lagueux, W.F. Broekaert, C. Hetru, J.A. Hoffmann Insect Immunity: septic injury of Drosophila induces the synthesis of a potent antifungal peptide with sequence homology to plant antifungal peptides Journal of Biological Chemistry, 269 (1994), pp. 33159–33163
- ↑ L. Michaut, P. Fehlbaum, M. Moniatte, A. Van Dorsselaer, J.M. Reichhart, P. Bulet Determination of the disulfide array of the first inducible antifungal peptide from insects: drosomycin from Drosophila melanogaster FEBS Letters, 395 (1996), pp. 6–10
- ↑ S. Uttenweiler-Joseph, M. Moniatte, M. Lagueux, A. Van Dorsselaer, J.A. Hoffmann, P. Bulet Differential display of peptides induced during the immune response of Drosophila: a matrix-assisted laser desorption ionization time-of-flight mass spectrometry study Proceedings of the National Academy of Sciences USA, 95 (1998), pp. 11342–11347
- ↑ Fant, Franky; Vranken, Wim; Broekaert, Willem; Borremans, Frans (1998-05-29). "Determination of the three-dimensional solution structure of Raphanus sativus Antifungal Protein 1 by 1H NMR1". Journal of Molecular Biology. 279 (1): 257–270. doi:10.1006/jmbi.1998.1767.
- ↑ Landon, Céline; Sodano, Patrick; Hetru, Charles; Hoffmann, Jules; Ptak, Marius (1997-09-01). "Solution structure of drosomycin, the first inducible antifungal protein from insects". Protein Science. 6 (9): 1878–1884. doi:10.1002/pro.5560060908. ISSN 1469-896X. PMC 2143780. PMID 9300487.
- ↑ "Drs Drosomycin [Drosophila melanogaster (fruit fly)] - Gene - NCBI". www.ncbi.nlm.nih.gov. Retrieved 2017-01-04.
- ↑ F. M. Jiggins and K. W. Kim, “The evolution of antifungal peptides in Drosophila,” Genetics, vol. 171, no. 4, pp. 1847– 1859, 2005
- ↑ W. Y. Yang, S. Y. Wen, Y. D. Huang, et al., “Functional divergence of six isoforms of antifungal peptide Drosomycin in Drosophila melanogaster,” Gene, vol. 379, pp. 26–32, 2006
- ↑ doi: 10.1038/srep32175
- ↑ Bulet, Phillipe; Hetru, Charles; Dimarcq, Jean-Luc; Hoffmann, Daniéle (1999-06-01). "Antimicrobial peptides in insects; structure and function". Developmental & Comparative Immunology. 23 (4–5): 329–344. doi:10.1016/S0145-305X(99)00015-4.