Disodium citrate
 | |
| Names | |
|---|---|
| IUPAC name
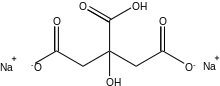
Disodium hydrogen 2-hydroxypropane-1,2,3-tricarboxylate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.005.113 |
| EC Number | 205-623-3 |
PubChem CID |
|
| RTECS number | GE7580000 |
| |
| |
| Properties | |
| C6H6Na2O7 | |
| Molar mass | 236.09 g·mol−1 |
| Appearance | white crystalline powder |
| Melting point | 149 °C (300 °F; 422 K) |
| Hazards | |
| NFPA 704 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Disodium citrate, more properly, disodium hydrogen citrate, is an acid salt of citric acid with the chemical formula Na2C6H6O7. It is used as an antioxidant in food and to improve the effects of other antioxidants.[1] It is also used as an acidity regulator and sequestrant.[1] Typical products include gelatin, jam, sweets, ice cream, carbonated beverages, milk powder, wine, and processed cheeses.
Disodium citrate may be used in patients to alleviate discomfort from urinary-tract infections.[2]
References
- 1 2 "Alkarate from Macleods: Disodium Hydrogen Citrate". drugsupdate.com.
- ↑ "OTC Treatment".
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.
