Digital microfluidics
Digital microfluidics (DMF) is another platform for lab-on-a-chip systems that is based upon the manipulation of microdroplets. Droplets are dispensed, moved, stored, mixed, reacted, or analyzed on a platform with a set of insulated electrodes.[1][2] Digital microfluidics can be used together with analytical analysis procedures such as mass spectrometry, colorimetry, electrochemical, and electrochemiluminescense.[1]
Overview
In analogy to digital microelectronics, digital microfluidic operations can be combined and reused within hierarchical design structures so that complex procedures (e.g. chemical synthesis or biological assays) can be built up step-by-step. And in contrast to continuous-flow microfluidics, digital microfluidics[3] works much the same way as traditional bench-top protocols, only with much smaller volumes and much higher automation. Thus a wide range of established chemical procedures and protocols can be seamlessly transferred to a nanoliter droplet format. Electrowetting, dielectrophoresis, and immiscible-fluid flows are the three most commonly used principles, which have been used to generate and manipulate microdroplets in a digital microfluidic device.
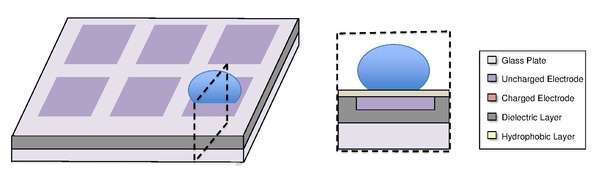
A digital microfluidic (DMF) device set-up depends on the substrates used, the electrodes, the configuration of those electrodes, the use of a dielectric material, the thickness of that dielectric material, the hydrophobic layers, and the applied voltage.[4][5]
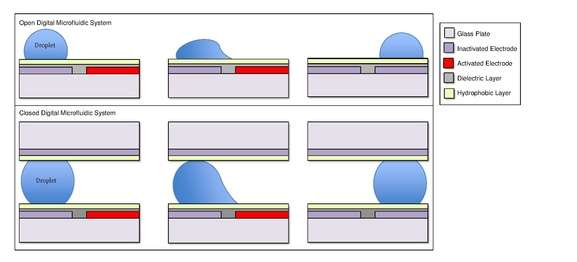
A common substrate used is this type of system is glass. Depending if the system is open or closed, there would be either one or two layers of glass. The bottom layer of the device contains a patterned array of individually controllable electrodes.[4] When looking at a closed system, there is usually a continuous ground electrode found through the top layer made usually of indium tin oxide (ITO). The dielectric layer is found around the electrodes in the bottom layer of the device and is important for building up charges and electrical field gradients on the device.[5] A hydrophobic layer is applied to the top layer of the system to decrease the surface energy where the droplet will actually we be in contact with.[5] The applied voltage activates the electrodes and allows changes in the wettability of droplet on the device’s surface. In order to move a droplet, a control voltage is applied to an electrode adjacent to the droplet, and at the same time, the electrode just under the droplet is deactivated. By varying the electric potential along a linear array of electrodes, electrowetting can be used to move droplets along this line of electrodes.[6]
Modifications to this foundation can also be fabricated into the basic design structure. One example of this is the addition of electrochemiluminescence detectors within the indium tin oxide layer (the ground electrode in a closed system) which aid in the detection of luminophores in droplets.[7] In general, different materials may also be used to replace basic components of a DMF system such as the use of PDMS instead of glass for the substrate.[8] Liquid materials can be added, such as oil or another substance, to a closed system to prevent evaporation of materials and decrease surface contamination.[6][9] Also, DMF systems can be compatible with ionic liquid droplets with the use of an oil in a closed device or with the use of a catena (a suspended wire) over an open DMF device.[9]
Digital microfluidics can be light-activated. Optoelectrowetting can be used to transport sessile droplets around a surface containing patterned photoconductors.[10] The photoelectrowetting effect[11] can also be used to achieve droplet transport on a silicon wafer without the necessity of patterned electrodes.[12]
Working principle
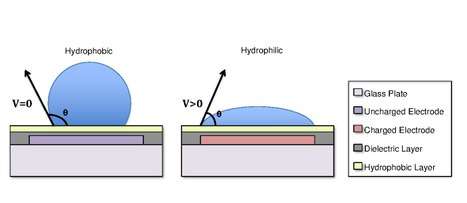
Droplets are formed using the surface tension properties of a liquid. For example, water placed on a hydrophobic surface such as wax paper will form spherical droplets to minimize its contact with the surface.[13] Differences in surface hydrophobicity affect a liquid’s ability to spread and ‘wet’ a surface by changing the contact angle.[14] As the hydrophobicity of a surface increases, the contact angle increases, and the ability of the droplet to wet the surface decreases. The change in contact angle, and therefore wetting, is regulated by the Young-Lipmann equation.[15][16][17]
where θ= contact angle with no voltage; θo= contact angles with applied voltage; εr=relative permittivity of the dielectric; ε0=permittivity of free space; V= applied voltage; γ= liquid/filler media surface tension; d= dielectric thickness.[17]
In some cases, the hydrophobicity of a substrate can be controlled by using electrical fields. This refers to the phenomenon Electrowetting On Dielectric (EWOD).[3][4][17] For example, when no electric field is applied to an electrode, the surface will remain hydrophobic and a liquid droplet will form a more spherical droplet with a greater contact angle. When an electric field is applied, a polarized hydrophilic surface is created. The water droplet then becomes flattened and the contact angle decreases. By controlling the localization of this polarization, we can create an interfacial tension gradient that allows controlled displacement of the droplet across the surface of the DMF device.[6]
Droplet Manipulation
Three-Dimensional Droplet Displacement by Electrostatic Actuation
Three-dimensional droplet actuation has been made possible by implementing a closed system; this system contains a µL sized droplet in immiscible fluid medium. The droplet and medium are then sandwiched between two electromagnetic plates, creating an EM field between the two plates.[18][19] The purpose of this method is to transfer the droplet from a lower planar surface to an upper parallel planar surface and back down via electrostatic forces.[18][20] The physics behind such particle actuation and perpendicular movement can be understood from early works of N. N. Lebedev and I. P. Skal’skaya.[21] In their research, they attempted to model the Maxwell electrical charge acquired by a perfectly round conducting particle in the presence of a uniform magnetic field caused by a perfectly-conducting and infinitely-stretching surface.[21] Their model helps to predict the Z-direction motion of the microdroplets within the device as it points to the magnitude and direction of forces acting upon a micro droplet. This can be used to help accurately predict and correct for unwanted and uncontrollable particle movement. The model explains why failing to employ dielectric coating on one of the two surfaces causes reversal of charge within the droplet upon contact with each electrode and in turn causes the droplets to uncontrollably bounce of between electrodes.
Digital microfluidics (DMF), has already been readily adapted in many biological fields.[22][23][24][25] By enabling three-dimensional movement within DMF, the technology can be used even more extensively in biological applications, as it could more accurately mimic 3-D microenvironments. A large benefit of employing this type of method is that it allows for two different environments to be accessible by the droplet, which can be taken advantage of by splitting the microfluidic tasks among the two surfaces. For example, while the lower plane can be used to move droplets, the upper plate can carry out the necessary chemical and/or biological processes.[18] This advantage can be translated into practical experiment protocols in the biological community, such as coupling with DNA amplification.[20] This also allows for the chip to be smaller, and to give researchers more freedom in designing platforms for microdroplet analysis.[18]
All-Terrain Droplet Actuation (ATDA)
All-terrain microfluidics is a method used to transport liquid droplets over non-traditional surface types.[26] Unlike traditional microfluidics platform, which are generally restricted to planar and horizontal surfaces, ATDA enables droplet manipulation over curved, non-horizontal, and inverted surfaces.[26] This is made possible by incorporating flexible thin sheets of copper and polyimide into the surface via a rapid prototyping method.[26][27] This device works very well with many liquids, including aqueous buffers, solutions of proteins and DNA, and undiluted bovine serum.[26] ATDA is compatible with silicone oil or pluronic additives, such as F-68, which reduce non-specific absorption and biofouling when dealing with biological fluids such as proteins, biological serums, and DNA.[26][28] A drawback of a setup like this is accelerated droplet evaporation.[26] ATDA is a form of open digital microfluidics, and as such the device needs to be encapsulated in a humidified environment in order to minimize droplet evaporation.[29]
Implementation
In one of various embodiments of EWOD-based microfluidic biochips, investigated first by Cytonix in 1987 and subsequently commercialized by Advanced Liquid Logic, there are two parallel glass plates. The bottom plate contains a patterned array of individually controllable electrodes and the top plate is coated with a continuous grounding electrode. A dielectric insulator coated with a hydrophobic is added to the plates to decrease the wet-ability of the surface and to add capacitance between the droplet and the control electrode. The droplet containing biochemical samples and the filler medium, such as the silicone oil, a fluorinated oil, or air, are sandwiched between the plates and the droplets travel inside the filler medium. In order to move a droplet, a control voltage is applied to an electrode adjacent to the droplet, and at the same time, the electrode just under the droplet is deactivated. By varying the electric potential along a linear array of electrodes, electrowetting can be used to move droplets along this line of electrodes.
Applications
Separation and Extraction
Digital microfluidics can be used for separation and extraction of target analytes. These methods include the use of magnetic particles,[30][31][32][33][34][35][36][37] liquid-liquid extraction,[38] optical tweezers,[39] and hydrodynamic effects.[40]
Magnetic Particles
For magnetic particle separations a droplet of solution containing the analyte of interest is placed on a digital microfluidics electrode array and moved by the changes in the charges of the electrodes. The droplet is moved to an electrode with a magnet on one side of the array with magnetic particles functionalized to bind to the analyte. Then it is moved over the electrode, the magnetic field is removed and the particles are suspended in the droplet. The droplet is swirled on the electrode array to ensure mixing. The magnet is reintroduced and the particles are immobilized and the droplet is moved away. This process is repeated with wash and elution buffers to extract the analyte.[30][31][32][33][34][35][36][37]
Magnetic particles coated with antihuman serum albumin antibodies have been used to isolate human serum albumin, as proof of concept work for immunoprecipitation using digital microfluidics.5 DNA extraction from a whole blood sample has also been performed with digital microfluidics.3 The procedure follows the general methodology as the magnetic particles, but includes pre-treatment on the digital microfluidic platform to lyse the cells prior to DNA extraction.[32]
Liquid-liquid Extraction
Liquid-liquid extractions can be carried out on digital microfluidic device by taking advantage of immiscible liquids.9 Two droplets, one containing the analyte in aqueous phase, and the other an immiscible ionic liquid are present on the electrode array. The two droplets are mixed and the ionic liquid extracts the analyte, and the droplets are easily separable.[38]
Optical Tweezers
Optical tweezers have also been used to separate cells in droplets. Two droplets are mixed on an electrode array, one containing the cells, and the other with nutrients or drugs. The droplets are mixed and then optical tweezers are used to move the cells to one side of the larger droplet before it is split.[41][39] For a more detailed explanation on the underlying principles, see Optical tweezers.
Hydrodynamic Separation
Particles have been applied for use outside of magnetic separation, with hydrodynamic forces to separate particles from the bulk of a droplet.[40] This is performed on electrode arrays with a central electrode and ‘slices’ of electrodes surrounding it. Droplets are added onto the array and swirled in a circular pattern, and the hydrodynamic forces from the swirling cause the particles to aggregate onto the central electrode.[40]
Biological Extraction
Biological separations usually involve low concentration high volume samples. This can pose an issue for digital microfluidics due to the small sample volume necessary.[33] Digital microfluidic systems can be combined with a macrofluidic system designed to decrease sample volume, in turn increasing analyte concentration.[33] It follows the same principles as the magnetic particles for separation, but includes pumping of the droplet to cycle a larger volume of fluid around the magnetic particles.[33] Extraction of drug analytes from dried urine samples has also been reported. A droplet of extraction solvent, in this case methanol, is repeatedly flowed over a sample of dried urine sample then moved to a final electrode where the liquid is extracted through a capillary and then analyzed using mass spectrometry.[42]
Digital Microfluidic Immunoassays
The advanced fluid handling capabilities of digital microfluidics (DMF) allows for the adoption of DMF as an immunoassay platform as DMF devices can precisely manipulate small quantities of liquid reagents. Both heterogeneous immunoassays (antigens interacting with immobilized antibodies) and homogeneous immunoassays (antigens interacting with antibodies in solution) have been developed using a DMF platform.[43] With regards to heterogeneous immunoassays, DMF can simplify the extended and intensive procedural steps by performing all delivery, mixing, incubation, and washing steps on the surface of the device (on-chip). Further, existing immunoassay techniques and methods, such as magnetic bead-based assays, ELISAs, and electrochemical detection, have been incorporated onto DMF immunoassay platforms.[44][45][46][47]
The incorporation of magnetic bead-based assays onto a DMF immunoassay platform has been demonstrated for the detection of multiple analytes, such as human insulin, IL-6, cardiac marker Troponin I (cTnI), thyroid stimulating hormone (TSH), sTNF-RI, and 17β-estradiol.[46][48][49][50] For example, a magnetic bead-based approached has been used for the detection of cTnI from whole blood in less than 8 minutes.[45] Briefly, magnetic beads containing primary antibodies were mixed with labeled secondary antibodies, incubated, and immobilized with a magnet for the washing steps. The droplet was then mixed with a chemiluminescent reagent and detection of the accompanying enzymatic reaction was measured on-chip with a photomultiplier tube.
The ELISA template, commonly used for performing immunoassays and other enzyme-based biochemical assays, has been adapted for use with the DMF platform for the detection of analytes such as IgE and IgG.[51][52] In one example,[44] a series of bioassays were conducted to establish the quantification capabilities of DMF devices, including an ELISA-based immunoassay for the detection of IgE. Superparamagnetic nanoparticles were immobilized with anti-IgE antibodies and fluorescently labeled aptamers to quantify IgE using an ELISA template. Similarly, for the detection of IgG, IgG can be immobilized onto a DMF chip, conjugated with horseradish-peroxidase (HRP)-labeled IgG, and then quantified through measurement of the color change associated with product formation of the reaction between HRP and tetramethylbenzidine.[51]
To further expand the capabilities and applications of DMF immunoassays beyond colorimetric detection (i.e., ELISA, magnetic bead-based assays), electrochemical detection tools (e.g., microelectrodes) have been incorporated into DMF chips for the detection of analytes such as TSH and rubella virus.[47][53][54] For example, Rackus et al.[53] integrated microelectrodes onto a DMF chip surface and substituted a previously reported chemiluminescent IgG immunoassay[55] with an electroactive species, enabling detection of rubella virus. They coated magnetic beads with rubella virus, anti-rubella IgG, and anti-human IgG coupled with alkaline phosphatase, which in turn catalyzed an electron transfer reaction that was detected by the on-chip microelectrodes.
Digital Microfluidics and Mass Spectrometry
The coupling of digital microfluidics (DMF) and Mass Spectrometry can largely be categorized into indirect off-line analysis, direct off-line analysis, and in-line analysis[56] and the main advantages of this coupling are decreased solvent and reagent use, as well as decreased analysis times.[57]
Indirect off-line analysis is the usage of DMF devices to combine reactants and isolate products, which are then removed and manually transferred to a mass spectrometer. This approach takes advantage of DMF for the sample preparation step but also introduces opportunities for contamination as manual intervention is required to transfer the sample. In one example of this technique, a Grieco three-component condensation was carried out on chip and was taken off the chip by micropipette for quenching and further analysis.[58]
Direct off-line analysis is the usage of DMF devices that have been fabricated and incorporated partially or totally into a mass spectrometer. This process is still considered off-line, however as some post-reaction procedures may be carried out manually (but on chip), without the use of the digital capabilities of the device. Such devices are most often used in conjugation with MALDI-MS. In MALDI-based direct off-line devices, the droplet must be dried and recrystallized along with matrix – operations that oftentimes require vacuum chambers.[56][59] The chip with crystallized analyte is then placed in to the MALDI-MS for analysis. One issue raised with MALDI-MS coupling to DMF is that the matrix necessary for MALDI-MS can be highly acidic, which may interfere with the on-chip reactions[60]
Inline analysis is the usage of devices that feed directly into mass spectrometers, thereby eliminating any manual manipulation. Inline analysis may require specially fabricated devices and connecting hardware between the device and the mass spectrometer.[56] Inline analysis is often coupled with electrospray ionization. In one example, a DMF chip was fabricated with a hole that led to a microchannel[61] This microchannel was, in turn, connected to an electrospray ionizer that emitted directly into a mass spectrometer. Another possible inline coupling is DMF and Surface Acoustic Wave (SAW) atomization. This technique utilizes the propagation of waves on flat piezoelectric surfaces to move and ionize droplets.[62] Some couplings utilize an external high-voltage pulse source at the physical inlet to the mass spectrometer [63] but the true role of such additions is uncertain.[64]
Nuclear Magnetic Resonance Spectroscopy
Nuclear magnetic resonance (NMR) spectroscopy can be used in conjunction with digital microfluidics (DMF) through the use of NMR microcoils, which are electromagnetic conducting coils that are less than 1 mm in size. Due to their size, these microcoils have several limitations, directly influencing the sensitivity of the machinery they operate within.
Microchannel/microcoil interfaces, previous to digital microfluidics, had several drawbacks such as in that many created large amounts of solvent waste and were easily contaminated.[65][66] In this way, the use of digital microfluidics and its capability to manipulate singlet droplets is promising.
The interface between digital microfluidics and NMR relaxometry has led to the creation of systems such as those used to detect and quantify the concentrations of specific molecules on microscales[66] with some such systems using two step processes in which DMF devices guide droplets to the NMR detection site.[67] Introductory systems of high-field NMR and 2D NMR in conjunction with microfluidics have also been developed.[65] These systems use single plate DMF devices with NMR microcoils in place of the second plate.
Chemical Synthesis in Digital Microfluidics
Digital Microfluidics (DMF) allows for precise manipulation and coordination in small-scale chemical synthesis reactions due to its ability to control micro scale volumes of liquid reagents, allowing for overall less reagent use and waste.[68] This technology can be used in the synthesis compounds such as peptidomimetics and PET tracers.[69][70][71] PET tracers require nanogram quantities and as such, DMF allows for automated and rapid synthesis of tracers with 90-95% efficiency compared to conventional macro-scale techniques.[70][72]
Organic reagents are not commonly used in DMF because they tend to wet the DMF device and cause flooding; however synthesis of organic reagents can be achieved through DMF techniques by carrying the organic reagents through an ionic liquid droplet, thus preventing the organic reagent from flooding the DMF device.[58] Droplets are combined together by inducing opposite charges thus attracting them to each other.[73] This allows for automated mixing of droplets. Mixing of droplets are also used to deposit MOF crystals for printing by delivering reagents into wells and evaporating the solutions for crystal deposition.[74] This method of MOF crystal deposition is relatively cheap and does not require extensive robotic equipment.[74]
DMF devices also can be used in cell cultures. Digital Microfluidic Immunocytochemistry in Single Cells (DISC) was developed using DMF platforms to culture and use antibodies to label phosphorylated proteins in the cell.[75] Cultured cells are then removed and taken off chip for screening. Another technique synthesizes hydrogels within DMF platforms. The process uses electrodes to deliver reagents to produce the hydrogel, and then delivery of cell culture reagents for absorption into the gel.[71][76] The hydrogels are an improvement over 2D cell culture because 3D cell culture have increased cell-cell interactions.[76] Spherical cell cultures are another method developed around the ability of DMF to deliver droplets to cells. Application of an electric potential allows for automation of droplet transfer directly to the hanging cell culture.[71][77] This is beneficial as cell culture in spheroids better mimic in vivo tissue.[77] Another use of DMF platforms in cell culture is its ability to conduct in vitro cell-free cloning using single molecule PCR inside droplets.[78] PCR amplified products are then cultured within the cell by using a temperature gradient across the surface of the DMF platform.[78]
References
- 1 2 Shamsi, Mohtashim H.; Choi, Kihwan; Ng, Alphonsus H. C.; Chamberlain, M. Dean; Wheeler, Aaron R. (2016-03-15). "Electrochemiluminescence on digital microfluidics for microRNA analysis". Biosensors & Bioelectronics. 77: 845–852. doi:10.1016/j.bios.2015.10.036. ISSN 1873-4235. PMID 26516684.
- ↑ "Duke Microfluidics Lab". microfluidics.ee.duke.edu. Retrieved 2017-05-22.
- ↑ C.-J. Kim, “Micropumping by Electrowetting”, Proc. ASME Int. Mechanical Engineering Congress and Exposition, New York, NY, Nov. 2001, IMECE2001/HTD-24200
- 1 2 Jain, Devarasetty, & Patrikar (2017). "Effect of electrode geometry on droplet velocity in open EWOD based device for digital microfluidics applications". Journal of Electrostatics. 87: 11–18. doi:10.1016/j.elstat.2017.02.006.
- 1 2 3 Choi, &; et al. (2012). "Digital Microfluidics". ARAC. 5: 413–440. doi:10.1146/annurev-anchem-062011-143028.
- 1 2 3 Fair, R. B.; Khlystov, A.; Tailor, T. D.; Ivanov, V.; Evans, R. D.; Srinivasan, V.; Pamula, V. K.; Pollack, M. G.; Griffin, P. B. (2007-01-01). "Chemical and Biological Applications of Digital-Microfluidic Devices". IEEE Design Test of Computers. 24 (1): 10–24. doi:10.1109/MDT.2007.8. ISSN 0740-7475.
- ↑ Shamsi, Mohtashim H.; Choi, Kihwan; Ng, Alphonsus H. C.; Chamberlain, M. Dean; Wheeler, Aaron R. (2016-03-15). "Electrochemiluminescence on digital microfluidics for microRNA analysis". Biosensors and Bioelectronics. 77: 845–852. doi:10.1016/j.bios.2015.10.036.
- ↑ Zhao, Y.; Xu, T.; Chakrabarty, K. (2011-07-01). "Broadcast Electrode-Addressing and Scheduling Methods for Pin-Constrained Digital Microfluidic Biochips". IEEE Transactions on Computer-Aided Design of Integrated Circuits and Systems. 30 (7): 986–999. doi:10.1109/TCAD.2011.2116250. ISSN 0278-0070.
- 1 2 Berthier, Jean (2008). Microdrops and digital microfluidics. William Andrew Pub. ISBN 9780815515449. OCLC 719878673.
- ↑ P.Y. Chiou, H. Moon, H. Toshiyoshi, C-J. Kim, M.C. Wu "Light actuation of liquid by optoelectrowetting" Sensors Actuator A 104, 222 (2003). doi:10.1016/S0924-4247(03)00024-4.
- ↑ S. Arscott "Moving liquids with light: Photoelectrowetting on semiconductors" Scientific Reports (Nature) 1, 184 (2011). doi:10.1038/srep00184.
- ↑ C. Palma and R.D. Deegan "Droplet Translation Actuated by Photoelectrowetting" Langmuir 34, 3177 (2018). doi:10.1021/acs.langmuir.7b03340.
- ↑ Goodman, Jeff. "Water Drops: Cohesion and Adhesion of Water". www.appstate.edu. Retrieved 2017-05-21.
- ↑ "Wetting". web.mit.edu. Retrieved 2017-05-21.
- ↑ Jain, Vandana; Devarasetty, Vasavi; Patrikar, Rajendra (2017-06-01). "Effect of electrode geometry on droplet velocity in open EWOD based device for digital microfluidics applications". Journal of Electrostatics. 87: 11–18. doi:10.1016/j.elstat.2017.02.006.
- ↑ 1952-, Berthier, Jean, (2008). Microdrops and digital microfluidics. William Andrew Pub. ISBN 9780815515449. OCLC 719878673.
- 1 2 3 Choi, Kihwan; Ng, Alphonsus H. C.; Fobel, Ryan; Wheeler, Aaron R. (2012-06-18). "Digital Microfluidics". dx.doi.org/10.1146/annurev-anchem-062011-143028. doi:10.1146/annurev-anchem-062011-143028. Retrieved 2017-05-21.
- 1 2 3 4 Roux, J. M., Fouillet, Y., & Achard, J. L. (2007). 3D droplet displacement in microfluidic systems by electrostatic actuation. Sensors and Actuators, A: Physical, 134(2), 486–493. https://doi.org/10.1016/j.sna.2006.05.012
- ↑ Fouillet, Y., & Achard, J. L. (2004). Microfluidique discrète et biotechnologie. Comptes Rendus Physique, 5(5), 577–588. https://doi.org/10.1016/j.crhy.2004.04.004
- 1 2 P.Kolar, R.B. Fair, Non-contact electrostatic stamping for DNA microarray synthesis (poster), in: Proceedings of the SmallTalk2001, San Diego, USA, 2001.
- 1 2 N.N. Lebedev, I.P. Skal’skaya, Force acting on a conducting sphere in the field of a parallel plate condenser, Soviet Phys. Tech. Phys. 7 (1962) 268–270.
- ↑ Velev, O. D., Prevo, B. G., & Bhatt, K. H. (2003). On-chip manipulation of free droplets. Nature, 426(6966), 515–516. https://doi.org/10.1038/426515a
- ↑ Gascoyne, P. R. C., Vykoukal, J. V, Schwartz, J. a, Anderson, T. J., Vykoukal, D. M., Current, K. W., … Andrews, C. (2004). Dielectrophoresis-based programmable fluidic processors. Lab on a Chip, 4(4), 299–309. https://doi.org/10.1039/b404130e
- ↑ Mukhopadhyay, R. (2006). Diving into droplets. Anal. Chem, 1401–1404.
- ↑ Taniguchi, T., Torii, T., & Higuchi, T. (2002). Chemical reactions in microdroplets by electrostatic manipulation of droplets in liquid media. Lab Chip, 2(1), 19–23. https://doi.org/10.1039/B108739H
- 1 2 3 4 5 6 Abdelgawad, M., Freire, S. L. S., Yang, H., & Wheeler, A. R. (2008). All-terrain droplet actuation. https://doi.org/10.1039/b801516c
- ↑ Abdelgawad, M., & Wheeler, A. R. (2007). Rapid prototyping in copper substrates for digital microfluidics. Advanced Materials, 19(1), 133–137. https://doi.org/10.1002/adma.200601818
- ↑ George, S. M., & Moon, H. (2015). Digital microfluidic three-dimensional cell culture and chemical screening platform using alginate hydrogels. Biomicrofluidics, 9(2), 1–13. https://doi.org/10.1063/1.4918377
- ↑ Barbulovic-Nad, I., Yang, H., Park, P. S., & Wheeler, A. R. (2008). Digital microfluidics for cell-based assays. Lab Chip, 8(4), 519–26. https://doi.org/10.1039/b717759c
- 1 2 Wang, Yizhong; Zhao, Yuejun; Cho, Sung Kwon (1 October 2007). "Efficient in-droplet separation of magnetic particles for digital microfluidics". Journal of Micromechanics and Microengineering. 17 (10): 2148–2156. doi:10.1088/0960-1317/17/10/029.
- 1 2 Vergauwe, Nicolas; Vermeir, Steven; Wacker, Josias B.; Ceyssens, Frederik; Cornaglia, Matteo; Puers, Robert; Gijs, Martin A.M.; Lammertyn, Jeroen; Witters, Daan (June 2014). "A highly efficient extraction protocol for magnetic particles on a digital microfluidic chip". Sensors and Actuators B: Chemical. 196: 282–291. doi:10.1016/j.snb.2014.01.076.
- 1 2 3 Seale, Brendon; Lam, Charis; Rackus, Darius G.; Chamberlain, M. Dean; Liu, Chang; Wheeler, Aaron R. (18 October 2016). "Digital Microfluidics for Immunoprecipitation". Analytical Chemistry. 88 (20): 10223–10230. doi:10.1021/acs.analchem.6b02915.
- 1 2 3 4 5 Shah, Gaurav J.; Kim, Chang-Jin CJ (April 2009). "Meniscus-Assisted High-Efficiency Magnetic Collection and Separation for EWOD Droplet Microfluidics". Journal of Microelectromechanical Systems. 18 (2): 363–375. doi:10.1109/JMEMS.2009.2013394.
- 1 2 Jebrail, Mais J.; Sinha, Anupama; Vellucci, Samantha; Renzi, Ronald F.; Ambriz, Cesar; Gondhalekar, Carmen; Schoeniger, Joseph S.; Patel, Kamlesh D.; Branda, Steven S. (15 April 2014). "World-to-Digital-Microfluidic Interface Enabling Extraction and Purification of RNA from Human Whole Blood". Analytical Chemistry. 86 (8): 3856–3862. doi:10.1021/ac404085p.
- 1 2 Hung, Ping-Yi; Jiang, Pei-Shing; Lee, Erh-Fang; Fan, Shih-Kang; Lu, Yen-Wen (5 April 2015). "Genomic DNA extraction from whole blood using a digital microfluidic (DMF) platform with magnetic beads". Microsystem Technologies. 23 (2): 313–320. doi:10.1007/s00542-015-2512-9.
- 1 2 Choi, Kihwan; Ng, Alphonsus H. C.; Fobel, Ryan; Chang-Yen, David A.; Yarnell, Lyle E.; Pearson, Elroy L.; Oleksak, Carl M.; Fischer, Andrew T.; Luoma, Robert P.; Robinson, John M.; Audet, Julie; Wheeler, Aaron R. (15 October 2013). "Automated Digital Microfluidic Platform for Magnetic-Particle-Based Immunoassays with Optimization by Design of Experiments". Analytical Chemistry. 85 (20): 9638–9646. doi:10.1021/ac401847x.
- 1 2 Choi, Kihwan; Boyacı, Ezel; Kim, Jihye; Seale, Brendon; Barrera-Arbelaez, Luis; Pawliszyn, Janusz; Wheeler, Aaron R. (April 2016). "A digital microfluidic interface between solid-phase microextraction and liquid chromatography–mass spectrometry". Journal of Chromatography A. 1444: 1–7. doi:10.1016/j.chroma.2016.03.029.
- 1 2 Wijethunga, Pavithra A. L.; Nanayakkara, Yasith S.; Kunchala, Praveen; Armstrong, Daniel W.; Moon, Hyejin (March 2011). "On-Chip Drop-to-Drop Liquid Microextraction Coupled with Real-Time Concentration Monitoring Technique". Analytical Chemistry. 83 (5): 1658–1664. doi:10.1021/ac102716s.
- 1 2 Shah, Gaurav J.; Ohta, Aaron T.; Chiou, Eric P.-Y.; Wu, Ming C.; Kim, Chang-Jin “CJ” (2009). "EWOD-driven droplet microfluidic device integrated with optoelectronic tweezers as an automated platform for cellular isolation and analysis". Lab on a Chip. 9 (12): 1732. doi:10.1039/b821508a.
- 1 2 3 Nejad, Hojatollah Rezaei; Samiei, Ehsan; Ahmadi, Ali; Hoorfar, Mina (2015). "Gravity-driven hydrodynamic particle separation in digital microfluidic systems". RSC Adv. 5 (45): 35966–35975. doi:10.1039/C5RA02068A.
- ↑ Neuman, Keir C.; Block, Steven M. (September 2004). "Optical trapping". Review of Scientific Instruments. 75 (9): 2787–2809. doi:10.1063/1.1785844. ISSN 0034-6748. PMC 1523313. PMID 16878180.
- ↑ Kirby, Andrea E.; Lafrenière, Nelson M.; Seale, Brendon; Hendricks, Paul I.; Cooks, R. Graham; Wheeler, Aaron R. (17 June 2014). "Analysis on the Go: Quantitation of Drugs of Abuse in Dried Urine with Digital Microfluidics and Miniature Mass Spectrometry". Analytical Chemistry. 86 (12): 6121–6129. doi:10.1021/ac5012969.
- ↑ Ng A.H.C., Uddayasankar U., Wheeler A.R. “Immunoassays in microfluidic systems”. Anal Bioanal Chem (2010) 39:991-1007. DOI: 10.1007/s00216-010-3678-8.
- 1 2 Vergauwe N., Witters D., Ceyssens F., Vermeir S., Verbruggen B., Puers R., Lammertyn J. “A versatile electrowetting-based digital microfluidic platform for quantitative homogeneous and heterogeneous bio-assays." J. Micromech. Microeng. 21 (2011) 054026. DOI: 10.1088/0960-1317/21/5/054026.
- 1 2 Sista R., Hua Z., Thwar P., Sudarsan A., Srinivasan V., Eckhardt A., Pollack M., Pamula V. “Development of a digital microfluidic platform for point of care testing”. Lab Chip, 2008, 8, 2091-2104. DOI: 10.1039/b814922d.
- 1 2 Ng A.H.C., Choi K., Luoma R.P., Robinson J.M., Wheeler A.R. “Digital Microfluidic Magnetic Separation for Particle-Based Immunoassays”. Anal. Chem. 2012, 84, 8805-8812. Dx.doi.org/10.1021/ac3020627.
- 1 2 Shamsi M.H., Choi K., Ng A.H.C., Wheeler A.R. “A digital microfluidic electrochemical immunoassay”. Lab Chip. 2014, 14, 547. DOI: 10.1039/c3lc51063h.
- ↑ Sista R.S., Eckhardt A.E., Srinivasan V., Pollack M.G., Palanki S., Pamula V.K. “Heterogeneous immunoassays using magnetic beads on a digital microfluidic platform.” Lab Chip. 2008, 8, 2188-2196. DOI: 10.1039/b807855f.
- ↑ Tsaloglou M.N., Jacobs A., Morgan H. “A fluorogenic heterogenous immunoassay for cardiac muscle troponin cTnI on a digital microfluidic device.” Anal Bioanal Chem. 2014, 406:5967-5976. DOI: 10.1007/s00216-014-7997-z.
- ↑ Huang C.Y., Tsai P.Y., Lee I.C., Hsu H.Y., Huang H.Y., Fan S.K., Yao D.J., Liu C.H., Hsu W. “A highly efficient bead extraction technique with low bead number for digital microfluidic immunoassay” Biomicro. 2016, 10, 011901. DOI: 10.1063/1.4939942.
- 1 2 Zhu L., Feng Y., Ye X., Feng J., Wu Y., Zhou Z. “An ELISA Chip Based on an EWOD Microfluidic Platform”. J. Adhesion Sci. Tech. 26, 2012, 2113-2124. DOI: 10.1163/156856111x600172.
- ↑ Miller E.M., Ng A.H.C., Uddayasankar U., Wheeler A.R. “A digital microfluidic approach to heterogeneous immunoassays.” Anal Bioanal Chem. 2011, 399:337-345. DOI: 10.1007/s00216-010-4368-2.
- 1 2 Rackus D.G., Dryden M.D.M., Lamanna J., Zaragoza A., Lam B., Kelley S.O., Wheeler A.R. “A digital microfluidic device with integrated nanostructured microelectrodes for electrochemical immunoassays”. Lab Chip. 2015, 15, 3776. DOI: 10.1039/c5lc00660k.
- ↑ Dixon C., Ng A.H.C., Fobel R., Miltenburg M.B., Wheeler A.R. “An inkjet printed, roll-coated digital microfluidic device for inexpensive, miniaturized diagnostic assays.” Lab Chip. 2016, 16, 4560. DOI: 10.1039/c6lc01064d.
- ↑ Ng A.H.C., Lee M., Choi K., Fischer A.T., Robinson J.M, Wheeler A.R. “Digital Microfluidic Platform for the Detection of Rubella Infection and Immunity: A Proof of Concept”. Clin Chem. 2015, 61:2, 420-429. DOI: 10.1373/clinchem.2014.232181
- 1 2 3 Kirby, Andrea E.; Wheeler, Aaron R. (2013-07-02). "Digital Microfluidics: An Emerging Sample Preparation Platform for Mass Spectrometry". Analytical Chemistry. 85 (13): 6178–6184. doi:10.1021/ac401150q. ISSN 0003-2700.
- ↑ Wang, Xue; Yi, Lian; Mukhitov, Nikita; Schrell, Adrian M.; Dhumpa, Raghuram; Roper, Michael G. (2015-02-20). "Microfluidics-to-mass spectrometry: A review of coupling methods and applications". Journal of Chromatography A. Editors' Choice IX. 1382: 98–116. doi:10.1016/j.chroma.2014.10.039. PMC 4318794. PMID 25458901.
- 1 2 Dubois, Philippe; Marchand, Gilles; Fouillet, Yves; Berthier, Jean; Douki, Thierry; Hassine, Fatima; Gmouh, Said; Vaultier, Michel (2006-07-01). "Ionic Liquid Droplet as e-Microreactor". Analytical Chemistry. 78 (14): 4909–4917. doi:10.1021/ac060481q. ISSN 0003-2700.
- ↑ Chatterjee, Debalina; Ytterberg, A. Jimmy; Son, Sang Uk; Loo, Joseph A.; Garrell, Robin L. (2010-03-01). "Integration of Protein Processing Steps on a Droplet Microfluidics Platform for MALDI-MS Analysis". Analytical Chemistry. 82 (5): 2095–2101. doi:10.1021/ac9029373. ISSN 0003-2700.
- ↑ Küster, Simon K.; Fagerer, Stephan R.; Verboket, Pascal E.; Eyer, Klaus; Jefimovs, Konstantins; Zenobi, Renato; Dittrich, Petra S. (2013-02-05). "Interfacing Droplet Microfluidics with Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry: Label-Free Content Analysis of Single Droplets". Analytical Chemistry. 85 (3): 1285–1289. doi:10.1021/ac3033189. ISSN 0003-2700.
- ↑ Jebrail, Mais J.; Yang, Hao; Mudrik, Jared M.; Lafrenière, Nelson M.; McRoberts, Christine; Al-Dirbashi, Osama Y.; Fisher, Lawrence; Chakraborty, Pranesh; Wheeler, Aaron R. (2011-09-05). "A digital microfluidic method for dried blood spot analysis". Lab on a Chip. 11 (19). doi:10.1039/c1lc20524b. ISSN 1473-0189.
- ↑ Yeo, Leslie Y.; Friend, James R. (2009-01-02). "Ultrafast microfluidics using surface acoustic waves". Biomicrofluidics. 3 (1): 012002. doi:10.1063/1.3056040. PMC 2717600. PMID 19693383.
- ↑ Heron, Scott R.; Wilson, Rab; Shaffer, Scott A.; Goodlett, David R.; Cooper, Jonathan M. (2010-05-15). "Surface Acoustic Wave Nebulization of Peptides As a Microfluidic Interface for Mass Spectrometry". Analytical Chemistry. 82 (10): 3985–3989. doi:10.1021/ac100372c. ISSN 0003-2700. PMC 3073871. PMID 20364823.
- ↑ Ho, Jenny; Tan, Ming K.; Go, David B.; Yeo, Leslie Y.; Friend, James R.; Chang, Hsueh-Chia (2011-05-01). "Paper-Based Microfluidic Surface Acoustic Wave Sample Delivery and Ionization Source for Rapid and Sensitive Ambient Mass Spectrometry". Analytical Chemistry. 83 (9): 3260–3266. doi:10.1021/ac200380q. ISSN 0003-2700.
- 1 2 Swyer, Ian; Soong, Ronald; Dryden, Michael D. M.; Fey, Michael; Maas, Werner E.; Simpson, André; Wheeler, Aaron R. (2016-11-01). "Interfacing digital microfluidics with high-field nuclear magnetic resonance spectroscopy". Lab Chip. 16 (22): 4424–4435. doi:10.1039/c6lc01073c. ISSN 1473-0189.
- 1 2 Lei, Ka-Meng; Mak, Pui-In; Law, Man-Kay; Martins, Rui P. (2015-07-13). "A palm-size μNMR relaxometer using a digital microfluidic device and a semiconductor transceiver for chemical/biological diagnosis". The Analyst. 140 (15): 5129–5137. doi:10.1039/c5an00500k. ISSN 1364-5528.
- ↑ Lei, Ka-Meng; Mak, Pui-In; Law, Man-Kay; Martins, Rui P. (2014-10-27). "NMR–DMF: a modular nuclear magnetic resonance–digital microfluidics system for biological assays". The Analyst. 139 (23): 6204–6213. doi:10.1039/c4an01285b. ISSN 1364-5528.
- ↑ Geng, Hongyao; Feng, Jian; Stabryla, Lisa Marie; Cho, Sung Kwon (2017-03-14). "Dielectrowetting manipulation for digital microfluidics: creating, transporting, splitting, and merging of droplets". Lab Chip. 17 (6): 1060–1068. doi:10.1039/c7lc00006e. ISSN 1473-0189.
- ↑ Jebrail, Mais J.; Assem, Naila; Mudrik, Jared M.; Dryden, Michael D.M.; Lin, Kaixiang; Yudin, Andrei K.; Wheeler, Aaron R. (2012-08-01). "Combinatorial Synthesis of Peptidomimetics Using Digital Microfluidics". Journal of Flow Chemistry. 2 (3): 103–107. doi:10.1556/JFC-D-12-00012. ISSN 2062-249X.
- 1 2 Chen, Supin; Javed, Muhammad Rashed; Kim, Hee-Kwon; Lei, Jack; Lazari, Mark; Shah, Gaurav J.; Dam, R. Michael van; Keng, Pei-Yuin; Kim, Chang-Jin “CJ” (2014-02-04). "Radiolabelling diverse positron emission tomography (PET) tracers using a single digital microfluidic reactor chip". Lab Chip. 14 (5): 902–910. doi:10.1039/c3lc51195b. ISSN 1473-0189.
- 1 2 3 Javed, Muhammad Rashed; Chen, Supin; Kim, Hee-Kwon; Wei, Liu; Czernin, Johannes; Kim, Chang-Jin “CJ”; Dam, R. Michael van; Keng, Pei Yuin (2014-02-01). "Efficient Radiosynthesis of 3′-Deoxy-3′-18F-Fluorothymidine Using Electrowetting-on-Dielectric Digital Microfluidic Chip". Journal of Nuclear Medicine. 55 (2): 321–328. doi:10.2967/jnumed.113.121053. ISSN 0161-5505. PMC 4494735. PMID 24365651.
- ↑ Keng, Pei Yuin; Chen, Supin; Ding, Huijiang; Sadeghi, Saman; Shah, Gaurav J.; Dooraghi, Alex; Phelps, Michael E.; Satyamurthy, Nagichettiar; Chatziioannou, Arion F. (2012-01-17). "Micro-chemical synthesis of molecular probes on an electronic microfluidic device". Proceedings of the National Academy of Sciences. 109 (3): 690–695. doi:10.1073/pnas.1117566109. ISSN 0027-8424. PMC 3271918. PMID 22210110.
- ↑ Um, Taewoong; Hong, Jiwoo; Im, Do Jin; Lee, Sang Joon; Kang, In Seok (2016-08-18). "Electrically Controllable Microparticle Synthesis and Digital Microfluidic Manipulation by Electric-Field-Induced Droplet Dispensing into Immiscible Fluids". Scientific Reports. 6 (1). doi:10.1038/srep31901. ISSN 2045-2322. PMC 4989170. PMID 27534580.
- 1 2 Witters, Daan (February 2012). "Digital Microfluidic High-Throughput Printing of Single Metal-Organic Framework Crystals". Wiley Online Library. 24: 1281–1346.
- ↑ Ng, Alphonsus H. C.; Chamberlain, M. Dean; Situ, Haozhong; Lee, Victor; Wheeler, Aaron R. (2015-06-24). "Digital microfluidic immunocytochemistry in single cells". Nature Communications. 6. doi:10.1038/ncomms8513. ISSN 2041-1723. PMC 4491823. PMID 26104298.
- 1 2 George, Subin M.; Moon, Hyejin (2015-03-01). "Digital microfluidic three-dimensional cell culture and chemical screening platform using alginate hydrogels". Biomicrofluidics. 9 (2): 024116. doi:10.1063/1.4918377. PMC 4401805. PMID 25945142.
- 1 2 Aijian, Andrew P.; Garrell, Robin L. (2014-12-15). "Digital Microfluidics for Automated Hanging Drop Cell Spheroid Culture". Journal of Laboratory Automation. 20 (3): 283–295. doi:10.1177/2211068214562002.
- 1 2 Yehezkel, Tuval Ben; Rival, Arnaud; Raz, Ofir; Cohen, Rafael; Marx, Zipora; Camara, Miguel; Dubern, Jean-Frédéric; Koch, Birgit; Heeb, Stephan (2016-02-29). "Synthesis and cell-free cloning of DNA libraries using programmable microfluidics". Nucleic Acids Research. 44 (4): e35–e35. doi:10.1093/nar/gkv1087. ISSN 0305-1048. PMC 4770201. PMID 26481354.