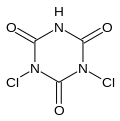
Dichloroisocyanuric acid
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1,3-dichloro-1,3,5-triazinane-2,4,6-trione | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.018.625 | ||
| KEGG | |||
PubChem CID |
|||
| |||
| |||
| Properties | |||
| C3HCl2N3O3 | |||
| Molar mass | 197.96 g/mol | ||
| Density | 2.2 g/cm3 | ||
| Melting point | 225 °C (437 °F; 498 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Dichloroisocyanuric acid, also known as dichlor or dichloro-s-triazinetrione and is marketed under many names (e.g. troclosene), is a chemical compound with the formula (C(O)NCl)2(C(O)NH).
Synthesis
Dichloroisocyanuric acid is manufactured by chlorination of cyanuric acid:[1]
- (C(O)NH)3 + 2 Cl2 → (C(O)NCl)2(C(O)NH) + 2 HCl
It is a colourless solid.
Mechanism of action
Dichloroisocyanuric acid is an oxidizer, reacting with water to form hypochlorous acid.
Although the bleaching agent in most chlorine based bleach is sodium hypochlorite, the sodium salt of dichloroisocyanuric acid, sodium dichloroisocyanurate, is the active ingredient in several bleach products. It is the active ingredient in many commercial disinfectant bacteriocides, algicides, and cleaning agents., for example the pulverized cleanser Comet.
See also
- Trichloroisocyanuric acid (trichlor)
References
- ↑ Huthmacher, K.; Most, D., "Cyanuric Acid and Cyanuric Chloride", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a08_191
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.