Desmopressin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | DDAVP (D-amino D-arginine vasopressin), Minrin, others |
| AHFS/Drugs.com | Monograph |
| Pregnancy category | |
| Routes of administration | IV, IM, SC, intranasal, by mouth, under the tongue |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Variable; 0.08–0.16% (by mouth) |
| Protein binding | 50% |
| Elimination half-life | 1.5–2.5 hours |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard |
100.037.009 |
| Chemical and physical data | |
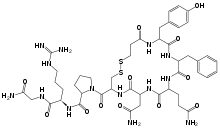
| Formula | C46H64N14O12S2 |
| Molar mass | 1069.22 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Desmopressin, sold under the trade name DDAVP among others, is a medication used to treat diabetes insipidus, bedwetting, hemophilia A, von Willebrand disease, and high blood urea levels.[1] In hemophilia A and von Willebrand disease, it should only be used for mild to moderate cases.[1] It may be given in the nose, by injection into a vein, by mouth, or under the tongue.[1]
Common side effects include headaches, diarrhea, and low blood sodium.[1] The low blood sodium that results may cause seizures.[1] It should not be used in people with significant kidney problems or low blood sodium.[1] It appears to be safe to use during pregnancy.[1] It is a synthetic version of vasopressin, the hormone that reduces urine production.[1]
Desmopressin was approved for medical use in the United States in 1978.[1] It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.[2] It is available as a generic medication.[1] In the United States a typical month supply is 100 to 200 USD.[3]
Medical uses
Bed wetting
Desmopressin is used to treat nocturnal enuresis (bedwetting). It is usually prescribed in the form of desmopressin acetate, by mouth. Children taking DDAVP have 2.2 fewer wet nights per week and are 4.5 times more likely to sleep without disruption compared with placebo.[4][5]
Nighttime urination
Desmopressin has some benefit in adults who have problems with night time urination (known as nocturia).[6] The FDA approved this use for those who make excess urine in 2017.[7]
Clotting disorders
Desmopressin (DDAVP) stimulates the release of von Willebrand factor (vWF) from the Weibel–Palade bodies of endothelial cells, thereby increasing the levels of vWF (as well as coagulant factor VIII) 3 to 5-fold. Desmopressin can be used to promote the release of von Willebrand factor (with subsequent increase in factor VIII survival secondary to vWF complexing) in patients with coagulation disorders such as von Willebrand disease, mild hemophilia A (factor VIII deficiency), and thrombocytopenia. It can be used with uremic induced platelet dysfunction. It is not effective in the treatment of hemophilia B (factor IX deficiency) or severe hemophilia A.
The usefulness of desmopressin in hemophilia A depends predominantly on whether the deficiency involved is qualitative (i.e., there is a functional mutation of the protein itself) or quantitative (i.e., there is decreased production of a functionally normal protein). Desmopressin is most effective in patients with quantitative deficiencies, because by increasing the release of vWF, the circulating pool of functional factor VIII effectively increases, as well. In patients who have functional defects in factor VIII, the use of desmopressin is moot, because it will only increase the circulating levels of a nonfunctional protein.
Diabetes insipidus
Desmopressin is used in the treatment of central diabetes insipidus (DI) as a replacement for endogenous antidiuretic hormone (ADH) that is in insufficient quantity due to decreased or non-existent secretion or production of ADH by the posterior pituitary or hypothalamus, respectively. It is also used in the diagnostic workup for diabetes insipidus, in order to distinguish central from DI due to the kidneys. Desmopressin is not effective at treating nephrogenic DI, thus a positive response is generally indicative of central DI.
Side effects
US drug regulators added warning to the nasal sprays after two people died and fifty-nine other people had seizures. This occurred due to hyponatremia, a deficit of the body's sodium levels, and the nasal spray is no longer approved for use in children in the United States.[8] However, US drug regulators have said that desmopressin tablets can still be considered safe for treatment of nocturnal enuresis in children as long as the person is otherwise healthy.
Patients must stop taking desmopressin if they develop severe vomiting and diarrhea, fever, the flu, or severe cold. Patients should also be very cautious about taking desmopressin during hot weather conditions or following strenuous exercise, as these conditions can place stress on the body's electrolyte and water balance.
A body needs to maintain a balance of water and (sodium). If sodium levels become too low (hyponatremia) – either as a result of increased water take-up or reduced salt levels – a person may have seizures and, in extreme cases, may die.[9]
Mechanism of action
Desmopressin works by limiting the amount of water that is eliminated in the urine; that is, it is an antidiuretic. It works at the level of the renal collecting duct by binding to V2 receptors, which signal for the translocation of aquaporin channels via cytosolic vesicles to the apical membrane of the collecting duct. The presence of these aquaporin channels in the distal nephron causes increasing water reabsorption from the urine, which becomes passively re-distributed from the nephron to systemic circulation by way of basolateral membrane channels.[10] Desmopressin also stimulates release of von Willebrand factor from endothelial cells by acting on the V2 receptor.
Desmopressin is degraded more slowly than recombinant vasopressin, and requires less frequent administration. In addition, it has little effect on blood pressure, while vasopressin may cause arterial hypertension. Vasopressin stimulates the release of ACTH, which indirectly increases responsiveness of alpha-1 receptor in blood vessel smooth muscle, increasing vessel tone and blood pressure. Several studies have shown that Desmopressin does not stimulate ACTH release (except in Cushing's Disease),[11][12][13] and therefore does not directly raise blood pressure, however, one study showed that it stimulates ACTH release in over 50% of healthy subjects.[14] Additionally, desmopressin is able to enhance ACTH and cortisol release in normal subjects following oCRH administration, but not in patients with anorexia nervosa.[13]
Chemistry
Desmopressin (1-deamino-8-D-arginine vasopressin) is a man-made form of the normal human hormone arginine vasopressin (the antidiuretic hormone, or ADH), a peptide containing nine amino acids.
Compared to vasopressin, desmopressin's first amino acid has been deaminated, and the arginine at the eighth position is in the dextro rather than the levo form (see stereochemistry).
References
- 1 2 3 4 5 6 7 8 9 10 "Desmopressin Acetate". The American Society of Health-System Pharmacists. Archived from the original on 3 December 2016. Retrieved 2 December 2016.
- ↑ "WHO Model List of Essential Medicines (19th List)" (PDF). World Health Organization. April 2015. Archived (PDF) from the original on 13 December 2016. Retrieved 8 December 2016.
- ↑ Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 233. ISBN 9781284057560.
- ↑ Evans, JH (2001). "Evidence based management of nocturnal enuresis". BMJ (Clinical research ed.). 323 (7322): 1167–9. doi:10.1136/bmj.323.7322.1167. PMC 1121645. PMID 11711411.
- ↑ "La prise en charge de l'énurésie nocturne primaire". Paediatr Child Health. 10 (10): 616–620. 2005. PMC 2722621. PMID 19668677.
- ↑ Ebell, MH; Radke, T; Gardner, J (Sep 2014). "A systematic review of the efficacy and safety of desmopressin for nocturia in adults". The Journal of Urology. 192 (3): 829–35. doi:10.1016/j.juro.2014.03.095. PMID 24704009.
- 1 2 Commissioner, Office of the. "Press Announcements - FDA approves first treatment for frequent urination at night due to overproduction of urine". www.fda.gov. Archived from the original on 2017-03-06.
- ↑ 2 Deaths Spur sleep apnea Drug Warning Archived 2007-12-07 at the Wayback Machine.. Webmd.com (2007-12-04). Retrieved on 2011-04-18.
- ↑ Archived December 13, 2007, at the Wayback Machine.
- ↑ Friedman FM, Weiss JP (December 2013). "Desmopressin in the treatment of nocturia: clinical evidence and experience". Ther Adv Urol. 5 (6): 310–7. doi:10.1177/1756287213502116. PMC 3825109. PMID 24294289.
- ↑ Pecori Giraldi, F; Marini, E; Torchiana, E; Mortini, P; Dubini, A; Cavagnini, F (June 2003). "Corticotrophin-releasing activity of desmopressin in Cushing's disease: lack of correlation between in vivo and in vitro responsiveness". The Journal of Endocrinology. 177 (3): 373–9. doi:10.1677/joe.0.1770373. PMID 12773117.
- ↑ Colombo, P; Passini, E; Re, T; Faglia, G; Ambrosi, B (June 1997). "Effect of desmopressin on ACTH and cortisol secretion in states of ACTH excess". Clinical endocrinology. 46 (6): 661–8. doi:10.1046/j.1365-2265.1997.1330954.x. PMID 9274696.
- 1 2 Foppiani, L; Sessarego, P; Valenti, S; Falivene, MR; Cuttica, CM; Giusti Disem, M (October 1996). "Lack of effect of desmopressin on ACTH and cortisol responses to ovine corticotropin-releasing hormone in anorexia nervosa". European journal of clinical investigation. 26 (10): 879–83. doi:10.1111/j.1365-2362.1996.tb02133.x. PMID 8911861.
- ↑ Scott, LV; Medbak, S; Dinan, TG (November 1999). "ACTH and cortisol release following intravenous desmopressin: a dose-response study". Clinical endocrinology. 51 (5): 653–8. doi:10.1046/j.1365-2265.1999.00850.x. PMID 10594528.
Further reading
- Leissinger, C; Becton, D; Cornell, C Jr; Cox Gill, J (2001). "High-dose DDAVP intranasal spray (Stimate) for the prevention and treatment of bleeding in patients with mild haemophilia A, mild or moderate type 1 von Willebrand disease and symptomatic carriers of haemophilia A.". Haemophilia. 7 (3): 258–66. doi:10.1046/j.1365-2516.2001.00500.x. PMID 11380629.