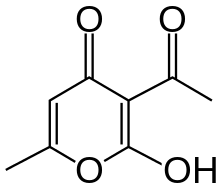
Dehydroacetic acid
 | |
| Names | |
|---|---|
| Preferred IUPAC name
3-Acetyl-2-hydroxy-6-methyl-4H-pyran-4-one | |
| Other names
Biocide 470F Methylacetopyronone | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | DHAA |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.541 |
| EC Number | 208-293-9 |
| E number | E265 (preservatives) |
| MeSH | dehydroacetic+acid |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C8H8O4 | |
| Molar mass | 168.15 g·mol−1 |
| Appearance | White crystals |
| Melting point | 109 °C; 228 °F; 382 K |
| Boiling point | 270 °C; 518 °F; 543 K |
| Hazards | |
EU classification (DSD) (outdated) |
|
| R-phrases (outdated) | R22 |
| S-phrases (outdated) | (S2) |
| NFPA 704 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dehydroacetic acid is an organic compound which has several industrial applications. The compound is classified as a pyrone derivative. It presents as an odorless, colorless to white crystalline powder, almost insoluble in water and moderately soluble in most organic solvents. [2]
Preparation
It is prepared by the base-catalysed dimerization of diketene.[3] Useful catalysts include the tertiary amines; imidazole, DABCO, and pyridine.[4]
Uses
Industrially, dehydroacetic acid has several uses which include the following:
- as a fungicide and bactericide. The sodium salt, sodium dehydroacetate, is often used in place of dehydroacetic acid because of its greater solubility in water.
- as a food preservative to prevent pickle bloating in squash and strawberries.[5] When used as a food additive, dehydroacetic acid is referred to using the International Numbering System for Food Additives or E number 265.
- as a plasticizer in synthetic resins.[1]
- as an antienzyme in toothpastes.
- as a precursor for dimethyl-4-pyridones. The compounds are synthesized when dehydroacetic acid is exposed to aqueous solutions containing primary amines.[6]
References
- 1 2 Merck Index, 11th Edition, 2855
- ↑ Jilalat, Alae Eddine et. al (2017). "DEHYDROACETIC ACID (Part 1): CHEMICAL AND PHARMACOLOGICAL PROPERTIES". Journal Marocain de Chimie Hétérocyclique. 16 (1): 1–47. ISSN 1114-7792. Retrieved July 3, 2017.
- ↑ Raimund Miller, Claudio Abaecherli, Adel Said, Barry Jackson. "Ketenes". In Ullmann's Encyclopedia of Industrial Chemistry. 2001, Wiley-VCH, Weinheim. doi: 10.1002/14356007.a15_063
- ↑ Clemens, Robert J.; Witzeman, J. Stewart (1993). Agreda, Victor H.; Zoeller, Joseph R., eds. Acetic Acid and its Derivatives. New York: Marcel Dekker, Inc. p. 202. ISBN 9780824787929.
- ↑ Harold William Rossmoore. Handbook of Biocide and Preservative Use, p. 341. ISBN 0-7514-0212-5
- ↑ Cook, Denys (1963). "The Preparation, Properties, and Structure of 2,6-bis-(Alkyamino)-2,5-heptadien-4-ones". Canadian Journal of Chemistry. 41 (6): 1435–1440. doi:10.1139/v63-195.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.
