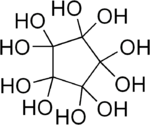
Decahydroxycyclopentane
 | |
| Names | |
|---|---|
| IUPAC name
cyclopentane-1,1,2,2,3,3,4,4,5,5-decaol | |
| Other names
decahydroxycyclopentane | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C5H10O10 | |
| Molar mass | 230.13 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Decahydroxycyclopentane is an organic compound with formula C5O10H10 or C5(OH)10. It is a fivefold geminal diol on a cyclopentane backbone.
The compound can be regarded as the fivefold hydrate of cyclopentanepentone. Indeed, the product referred to in the literature and trade as "cyclopentanepentone pentahydrate" (C5O5·5H2O) is now believed to be the decahydroxycyclopentane.[1][2]
The compound was synthetized by Heinrich Will in 1861, although for a long time it was believed to be a hydrate of C5O5. It can be prepared by oxidation of croconic acid C5O3(OH)2 with nitric acid.[3] It can be isolated as water-soluble colorless crystals that melt with dehydration at about 115 °C, and slowly decompose at about 160 °C.[4]
References
- ↑ Gunther Seitz; Peter Imming (1992). "Oxocarbons and pseudooxocarbons". Chemical Reviews. 92 (6): 1227–1260. doi:10.1021/cr00014a004.
- ↑ Willis B. Person and Dale G. Williams (1957), "Infrared spectra and the structures of leuconic acid and triquinoyl". J. Phys. Chem., 61 (7), 1017-1018. doi:10.1021/j150553a047
- ↑ Will, H. (1861). "Beitrag zur Kenntniss der Krokonsäure". Justus Liebigs Annalen der Chemie. 118 (2): 177&ndash, 187. doi:10.1002/jlac.18611180204.
- ↑ Fatiadi, Alexander J.; Horace S. Isbell; William F. Sager (March–April 1963). "Cyclic Polyhydroxy Ketones. I. Oxidation Products of Hexahydroxybenzene (Benzenehexol)" (PDF). Journal of Research of the National Bureau of Standards Section A. 67A (2): 153&ndash, 162. doi:10.6028/jres.067A.015.
See also
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.