Dipeptidyl peptidase 8 is an enzyme that in humans is encoded by the DPP8 gene.[5][6]
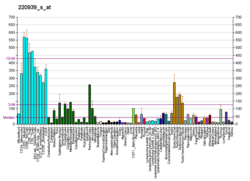
This gene encodes a member of the peptidase S9B family, a small family of dipeptidyl peptidases that are able to cleave peptide substrates at a prolyl bond. The encoded protein shares similarity with dipeptidyl peptidase IV in that it is ubiquitously expressed, and hydrolyzes the same substrates. These similarities suggest that, like dipeptidyl peptidase IV, this protein may play a role in T-cell activation and immune function. Alternatively spliced transcript variants encoding different isoforms have been described.[6]
References
- Notes
- Further reading
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Ajami K, Abbott CA, Obradovic M, et al. (2003). "Structural requirements for catalysis, expression, and dimerization in the CD26/DPIV gene family". Biochemistry. 42 (3): 694–701. doi:10.1021/bi026846s. PMID 12534281.
- Qi SY, Riviere PJ, Trojnar J, et al. (2003). "Cloning and characterization of dipeptidyl peptidase 10, a new member of an emerging subgroup of serine proteases". Biochem. J. 373 (Pt 1): 179–89. doi:10.1042/BJ20021914. PMC 1223468. PMID 12662155.
- Ota T, Suzuki Y, Nishikawa T, et al. (2004). "Complete sequencing and characterization of 21,243 full-length human cDNAs". Nat. Genet. 36 (1): 40–5. doi:10.1038/ng1285. PMID 14702039.
- Shu H, Chen S, Bi Q, et al. (2004). "Identification of phosphoproteins and their phosphorylation sites in the WEHI-231 B lymphoma cell line". Mol. Cell. Proteomics. 3 (3): 279–86. doi:10.1074/mcp.D300003-MCP200. PMID 14729942.
- Chen YS, Chien CH, Goparaju CM, et al. (2004). "Purification and characterization of human prolyl dipeptidase DPP8 in Sf9 insect cells". Protein Expr. Purif. 35 (1): 142–6. doi:10.1016/j.pep.2003.12.019. PMID 15039077.
- Gerhard DS, Wagner L, Feingold EA, et al. (2004). "The Status, Quality, and Expansion of the NIH Full-Length cDNA Project: The Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334.
- Jiaang WT, Chen YS, Hsu T, et al. (2005). "Novel isoindoline compounds for potent and selective inhibition of prolyl dipeptidase DPP8". Bioorg. Med. Chem. Lett. 15 (3): 687–91. doi:10.1016/j.bmcl.2004.11.023. PMID 15664838.
- Ogasawara W, Tanaka C, Suzuki M, et al. (2005). "Isoforms of dipeptidyl aminopeptidase IV from Pseudomonas sp. WO24: role of the signal sequence and overexpression in Escherichia coli". Protein Expr. Purif. 41 (2): 241–51. doi:10.1016/j.pep.2004.10.027. PMID 15866709.
- Kimura K, Wakamatsu A, Suzuki Y, et al. (2006). "Diversification of transcriptional modulation: Large-scale identification and characterization of putative alternative promoters of human genes". Genome Res. 16 (1): 55–65. doi:10.1101/gr.4039406. PMC 1356129. PMID 16344560.
- Yu DM, Wang XM, Ajami K, et al. (2006). "DP8 and DP9 have extra-enzymatic roles in cell adhesion, migration and apoptosis". Adv. Exp. Med. Biol. Advances in Experimental Medicine and Biology. 575: 63–72. doi:10.1007/0-387-32824-6_7. ISBN 978-0-387-29058-4. PMID 16700509.
- Yu DM, Wang XM, McCaughan GW, Gorrell MD (2006). "Extraenzymatic functions of the dipeptidyl peptidase IV-related proteins DP8 and DP9 in cell adhesion, migration and apoptosis". FEBS J. 273 (11): 2447–60. doi:10.1111/j.1742-4658.2006.05253.x. PMID 16704418.
- Lee HJ, Chen YS, Chou CY, et al. (2007). "Investigation of the dimer interface and substrate specificity of prolyl dipeptidase DPP8". J. Biol. Chem. 281 (50): 38653–62. doi:10.1074/jbc.M603895200. PMID 17040910.