Cytochrome c family
| Cytochrome c | |||||||||
|---|---|---|---|---|---|---|---|---|---|
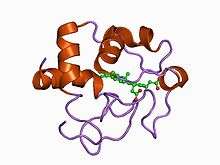 Structure of cytochrome c2 from Rhodopseudomonas viridis.[1] | |||||||||
| Identifiers | |||||||||
| Symbol | Cytochrom_C | ||||||||
| Pfam | PF00034 | ||||||||
| InterPro | IPR003088 | ||||||||
| PROSITE | PDOC00169 | ||||||||
| SCOP | 1cry | ||||||||
| SUPERFAMILY | 1cry | ||||||||
| OPM superfamily | 71 | ||||||||
| OPM protein | 1hrc | ||||||||
| Membranome | 210 | ||||||||
| |||||||||
| Cytochrome C' | |||||||||
|---|---|---|---|---|---|---|---|---|---|
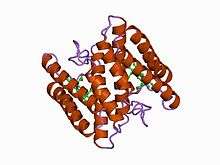 atomic structure of a cytochrome c' with an unusual ligand-controlled dimer dissociation at 1.8 angstroms resolution | |||||||||
| Identifiers | |||||||||
| Symbol | Cytochrom_C_2 | ||||||||
| Pfam | PF01322 | ||||||||
| InterPro | IPR002321 | ||||||||
| PROSITE | PDOC00169 | ||||||||
| SCOP | 1cgo | ||||||||
| SUPERFAMILY | 1cgo | ||||||||
| |||||||||
| Class III cytochrome C family | |||||||||
|---|---|---|---|---|---|---|---|---|---|
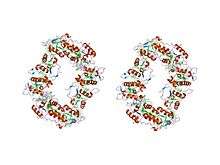 sulfate respiration in desulfovibrio vulgaris hildenborough: structure of the 16-heme cytochrome c hmca at 2.5 a resolution and a view of its role in transmembrane electron transfer | |||||||||
| Identifiers | |||||||||
| Symbol | Cytochrom_CIII | ||||||||
| Pfam | PF02085 | ||||||||
| Pfam clan | CL0317 | ||||||||
| InterPro | IPR020942 | ||||||||
| SCOP | 2cdv | ||||||||
| SUPERFAMILY | 2cdv | ||||||||
| CDD | cd08168 | ||||||||
| |||||||||
Cytochromes c (cytC) are electron-transfer proteins having one or several heme c groups, bound to the protein by one or, more generally, two thioether bonds involving sulphydryl groups of cysteine residues. The fifth haem iron ligand is always provided by a histidine residue. Cytochromes c possess a wide range of properties and function in a large number of different redox processes.[2] The founding member of this family is mitochondrial cytochrome c.
Ambler[3] recognized four classes of cytC.
- Class I includes the low-spin soluble cytC of mitochondria and bacteria, with the haem-attachment site towards the N-terminus, and the sixth ligand provided by a methionine residue about 40 residues further on towards the C-terminus. On the basis of sequence similarity, class I cytC were further subdivided into five classes, IA to IE. Class IB includes the eukaryotic mitochondrial cytC and prokaryotic 'short' cyt c2 exemplified by Rhodopila globiformis cyt c2; class IA includes 'long' cyt c2, such as Rhodospirillum rubrum cyt c2 and Aquaspirillum itersonii cytc-550, which have several extra loops by comparison with class IB cytC.
- Class II includes the high-spin cytC' and a number of low-spin cytochromes, e.g. cyt c-556. The haem-attachment site is close to the C terminus. The cytC' are capable of binding such ligands as CO, NO or CN(-), albeit with rate and equilibrium constants 100 to 1,000,000-fold smaller than other high-spin haemoproteins.[4] This, coupled with its relatively low redox potential, makes it unlikely that cytC' is a terminal oxidase. Thus cytC' probably functions as an electron transfer protein.[5] The 3D structures of a number of cytC' have been determined. The molecule usually exists as a dimer, each monomer folding as a four-alpha-helix bundle incorporating a covalently-bound haem group at the core.[5] The Chromatium vinosum cytC' exhibits dimer dissociation upon ligand binding.[6]
- Class III comprises the low redox potential multiple haem cytochromes: cyt C7 (trihaem), C3 (tetrahaem), and high-molecular-weight cytC, HMC (hexadecahaem), with only 30-40 residues per haem group. The haem c groups, all bis-histidinyl coordinated, are structurally and functionally nonequivalent and present different redox potentials in the range 0 to -400 mV.[7] The 3D structures of a number of cyt C3 proteins have been determined. The proteins consist of 4-5 alpha-helices and 2 beta-strands wrapped around a compact core of four non-parallel haems, which present a relatively high degree of exposure to the solvent. The overall protein architecture, haem plane orientations and iron-iron distances are highly conserved.[7]
- Class IV includes complex proteins containing other prosthetic groups besides haem c, such as flavocytochromes c and cytochromes cd.[3]
Subfamilies
Human proteins containing this domain
CYCS;
References
- ↑ Miki K, Sogabe S, Uno A, et al. (May 1994). "Application of an automatic molecular-replacement procedure to crystal structure analysis of cytochrome c2 from Rhodopseudomonas viridis". Acta Crystallogr. D. 50 (Pt 3): 271–5. doi:10.1107/S0907444993013952. PMID 15299438.
- ↑ Pettigrew GW, Moore GR (1987). "The Function of Bacterial and Photosynthetic Cytochromes c". Cytochromes c : Biological Aspects. Berlin Heidelberg: Springer. pp. 113–229. doi:10.1007/978-3-642-72698-9_3. ISBN 978-3-642-72698-9.
- 1 2 Ambler RP (1991). "Sequence variability in bacterial cytochromes c". Biochim. Biophys. Acta. 1058 (1): 42–47. doi:10.1016/S0005-2728(05)80266-X. PMID 1646017.
- ↑ Kassner RJ (May 1991). "Ligand binding properties of cytochromes c'". Biochim. Biophys. Acta. 1058 (1): 8–12. doi:10.1016/s0005-2728(05)80257-9. PMID 1646027.
- 1 2 Moore GR (May 1991). "Bacterial 4-alpha-helical bundle cytochromes". Biochim. Biophys. Acta. 1058 (1): 38–41. doi:10.1016/s0005-2728(05)80265-8. PMID 1646016.
- ↑ Ren Z, Meyer T, McRee DE (November 1993). "Atomic structure of a cytochrome c' with an unusual ligand-controlled dimer dissociation at 1.8 A resolution". J. Mol. Biol. 234 (2): 433–45. doi:10.1006/jmbi.1993.1597. PMID 8230224.
- 1 2 Coutinho IB, Xavier AV (1994). "Tetraheme cytochromes". Meth. Enzymol. 243: 119–40. doi:10.1016/0076-6879(94)43011-X. PMID 7830606.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.