Cryogenic oxygen plant
A cryogenic oxygen plant is an industrial facility that creates molecular oxygen at relatively high purity. Oxygen is the most common element in the earth's crust[1] and the second largest industrial gas. This process was pioneered by Dr. Carl von Linde in 1902.
Purpose
The cryogenic air separation achieves high purity oxygen of more than 99.5%. The resulting high purity product can be stored as a liquid and/or filled into cylinders. These cylinders can even be distributed to customer in the medical sector, welding or mixed with other gases and used as breathing gas for diving. Typical production ranges from 50 normal m3/hour up to 860,000 Nm3/hour (Ras Laffan refinery).
Plant modules
A cryogenic oxygen plant comprises:
Warm end (W/E) container
- Compressor
- Air receiver
- Chiller (Heat exchanger)
- Pre-filter
- Air purification unit (APU)
Coldbox
- Main heat exchanger
- Boiler
- Distillation column
- Expansion brake turbine
Storage
- Liquid oxygen tank
- Vapouriser
- Filling station
How the plant works
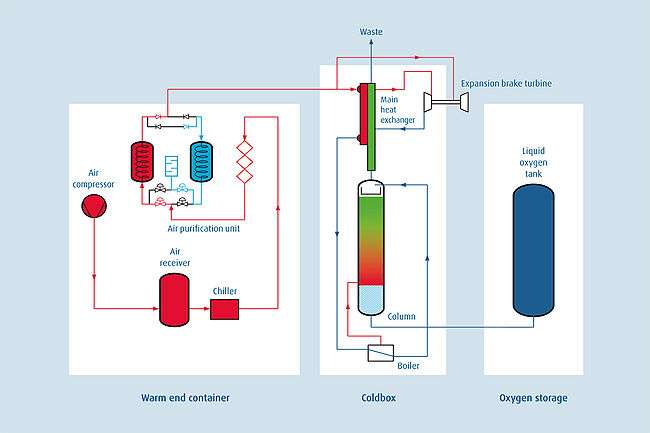
Warm end process
Atmospheric air is roughly filtered and pressurised by a compressor, which provides the product pressure to deliver to the customer. The amount of air sucked in depends on the customer’s oxygen demand.
The air receiver collects condensate and minimises pressure drop. The dry and compressed air leaves the air to refrigerant heat exchanger with about 10°C.
To clean the process air further, there are different stages of filtration. First of all, more condensate is removed, then a coalescing filter acts as a gravity filter and finally an adsorber filled with activated carbon removes some hydrocarbons.
The last unit process in the warm end container is the thermal swing adsorber (TSA). The Air purification unit cleans the compressed process air by removing any residual water vapour, carbon dioxide and hydrocarbons. It comprises two vessels, valves and exhaust to allow the changeover of vessels. While one of the TSA beds is on stream the second one is regenerated by the waste gas flow, which is vented through a silencer into the ambient environment.
Coldbox process
The process air enters the main heat exchanger in the coldbox where it is cooled in counter flow with the waste gas stream. After leaving the main heat exchanger the process air has a temperature of about –112°C and is partly liquefied. The complete liquefaction is achieved through evaporation of cooled liquid oxygen in the boiler. After passing a purity control valve process air enters on tip of the distillation column and flows down through the packing material.
The steam of evaporated oxygen vapour in the shell of the boiler vents back into the distillation column. It rises through the column packing material and encounters the descending stream of liquid process air.
The liquid air descending down the column loses nitrogen. It becomes richer in oxygen and collects at the base of the column as pure liquid oxygen. It flows out into the boiler to the cold box liquid product valve. An on-line oxygen analyser controls the opening of the liquid product valve to transfer pure low-pressure liquid oxygen into the storage tank.
The rising oxygen vapour becomes rich in nitrogen and argon. It leaves the column and exits the cold box at ambient temperature through the main heat exchanger as a waste gas. This waste gas provides purge gas to regenerate the TSA unit and to the cool the refrigeration turbine.
Turbines located at the base of the cold box provide refrigeration for the process. A stream of high-pressure gas from the main heat exchangers is cooled and expanded to low pressure in the turbine. This cold air returns to the waste stream of the heat exchanger to inject refrigeration. Energy removed by the turbine re-appears as heat in the turbine’s closed-cycle air-brake circuit. This heat is removed in an air-to-air cooler by waste gas from the cold box.
Storage and vaporising process
Liquid from the tank is compressed to high pressure in a cryogenic liquid pump. It is then vaporised in an ambient air evaporator to produce gaseous oxygen. The high-pressure gas then can pass into cylinders via the gas manifold or fed into a customers product pipeline.
Applications
- Furnace enrichment
- Medical gases
- Metal production
- Welding
See also
References
- ↑ Nave, R. Abundances of the Elements in the Earth's Crust, Georgia State University