Corey–Winter olefin synthesis
The Corey–Winter olefin synthesis is a series of chemical reactions for converting 1,2-diols into olefins.[1][2] It is named for the American chemist and Nobelist Elias James Corey and the American-Estonian chemist Roland Arthur Edwin Winter.[3]

Often, thiocarbonyldiimidazole is used instead of thiophosgene as shown above, since thiophosgene has a similar toxicity profile as phosgene, whereas thiocarbonyldiimidazole is a much safer alternative.
Mechanism
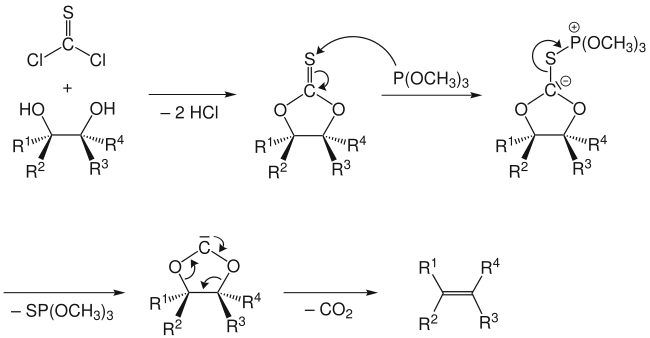
The reaction mechanism involves the formation of a cyclic thiocarbonate from the diol and thiophosgene. The second step involves treatment with trimethyl phosphite, which attacks the sulfur atom, producing S=P(OMe)3 (driven by the formation of a strong P=S double bond) and leaving a carbene.[4] This carbene collapses with loss of carbon dioxide to give the olefin.
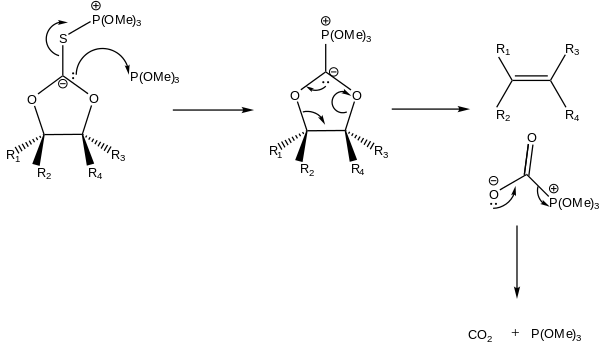
An alternative mechanism does not involve a free carbene intermediate, but rather involves attack of the carbanion by a second molecule of trimethylphosphite with concomitant cleavage of the sulfur-carbon bond. The phosphorus stabilized carbanion then undergoes an elimination to give the alkene, along with an acyl phosphite, which then decarboxylates.
The Corey-Winter olefination is a stereospecific reaction: a trans-diol gives a trans-alkene, while a cis-diol gives a cis-alkene as the product.
References
- ↑ Corey, E. J.; Winter, R. A. E. J. Am. Chem. Soc. 1963, 85, 2677. (doi:10.1021/ja00900a043)
- ↑ Corey, E. J.; Hopkiss, P. B. Tetrahedron Lett. 1982, 23, 1979. (doi:10.1016/S0040-4039(00)87238-X)
- ↑ Block, E. Org. React. 1984, 30, 457. doi:10.1002/0471264180.or030.02
- ↑ Horton, D.; Tindall, Jr., C. G. J. Org. Chem. 1970, 35(10), 3558-3559. (doi:10.1021/jo00835a082)