Cook–Heilbron thiazole synthesis

The Cook–Heilbron thiazole synthesis highlights the formation of 5-aminothiazoles through the chemical reaction of α-aminonitriles or aminocynoacetates with dithioacids, carbon disulphide, carbon oxysulfide, or isothiocynates at room temperature and under mild or aqueous conditions.[1][2] Variation of substituents at the 2nd and 4th position of the thiazole is introduced by selecting different combinations of starting reagents.[2]

This reaction was first discovered in 1947 by Alan H. Cook, Sir Ian Heilbron, and A.L Levy, and marks one of the first examples of 5-aminothiazole synthesis with significant yield and diversity in scope.[1] Prior to their discovery, 5-aminothiazoles were a relatively unknown class of compounds, but were of synthetic interest and utility.[1][3] Their premier publication illustrated the formation of 5-amino-2-benzylthiazole and 5-amino-4-carbethoxy-2-benzylthiazole by reacting dithiophenylacetic acid with aminoacetonitrile and ethyl aminocyanoacetate, respectively.[1] Subsequent experiments by Cook and Heilbron, detailed in their series of publications titled “Studies in the Azole Series” describe early attempts to expand the scope of 5-aminothiazole synthesis, as well as employ 5-aminothiazoles in the formation of purines and pyridines.[3][4][5][5][6]
| Cook-Heilbron thiazole synthesis | |
|---|---|
| Named after | Alan H. Cook Ian Heilbron |
| Reaction type | Ring forming reaction |
Mechanism

In the first step of the reaction mechanism for the synthesis of a 5-aminothiazole from an α-aminonitrile and carbon disulphide, a lone pair on the nitrogen of the α-aminonitrile[7] performs a nucleophilic attack on the slightly electropositive carbon of carbondisulfide. This addition reaction pushes electrons from the carbon-sulfur double bond onto one of the sulfur atoms. Acting as a Lewis Base, the sulfur atom donates its electrons to the carbon atom of the nitrile, forming a sulfur-carbon sigma bond in an intramolecular 5-exo-dig cyclization. This cyclization forms a 5-imino-2-thione thiazolidine compound that undergoes a tautomerization when a base, such as water, abstracts the hydrogens at positions 3 and 4. The electrons from the carbon-hydrogen sigma bond are pushed back into the thiazole ring, forming two new double bonds with the adjacent carbon atoms, and catalyzing the formation of two new nitrogen-hydrogen, and sulfur-hydrogen sigma bonds. This tautomerization occurs because it is thermodynamically favourable, yielding the aromatic final product: 5-aminothiazole.
Applications
Few instances of applications of the Cook–Heilbron thiazole synthesis are found in literature.[2] In recent years, modifications of the Hantzsch thiazole synthesis are the most common, partly because of its ease in introducing R- group diversity.[8]
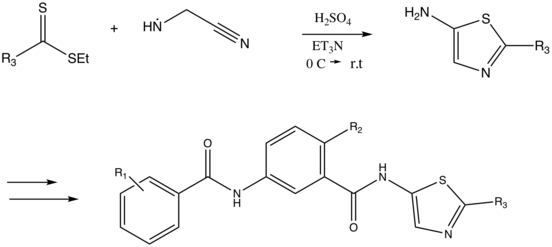
However, in 2008 Scott et al. employed a Cook-Heilbron synthesis in their approach to synthesize novel of pyridyl and thiazoyl bisamide CSF-1R inhibitors for use in novel cancer therapeutics.[9] A couple of the compounds that were analysed for in vivo anti-cancer activity contained thiazole derivatives that had been synthesized using a Cook-Heilbron approach. For instance, 2-methyl-5-aminothiazoles were prepared via condensation and cyclization of aminoacetonitrile and ethyldithioacetate as part of the synthesis of thiazolyl bisamines:[9]
Relevance
Thiazoles are essential components of many biologically active compounds making them important features in drug design.[10] Thiazoles are found in a number of pharmacological compounds such as tiazofurin and dasatinib (antineoplastic agents), ritonavir (an anti-HIV drug), ravuconazole (antifungal agent), meloxicam and fentiazac (anti-inflammatory agents) and nizatidine (anti-ulcer agent).[10]
Consequently, understanding and applying a range of approaches to synthesize thiazoles facilitates greater flexibility in both designing drugs as well as optimizing synthetic routes.
References
- 1 2 3 4 Cook, A. H; Heilbron, Ian (1947). "Studies in the azole series. Part I. A novel route to 5-aminothiazoles". J. Chem. Soc. 0: 1594–1598 – via Royal Society of Chemistry.
- 1 2 3 Li, J (2013). Heterocyclic Chemistry in Drug Discovery. Hoboken, N.J.: Wiley.
- 1 2 Cook, A. H.; Heilbron, Ian; Levy, A. L. (1947). "319. Studies in the azole series. Part II. The interaction of α-amino-nitriles and carbon disulphide". J. Chem. Soc. 0 (0): 1598–1609. doi:10.1039/jr9470001598. ISSN 0368-1769.
- ↑ Cook, A. H.; Heilbron, Ian; Mahadevan, A. P. (1949). "225. Studies in the azole series. Part XI. The interaction of α-amino-nitriles, hydrogen sulphide, and ketones". J. Chem. Soc. 0 (0): 1061–1064. doi:10.1039/jr9490001061. ISSN 0368-1769.
- 1 2 Cook, A. H.; Heilbron, Ian; Macdonald, S. F.; Mahadevan, A. P. (1949). "226. Studies in the azole series. Part XII. Some thiazolopyrimidines". Journal of the Chemical Society (Resumed). 0 (0): 1064. doi:10.1039/jr9490001064. ISSN 0368-1769.
- ↑ Cook, A. H.; Davis, A. C.; Heilbron, Ian; Thomas, G. H. (1949). "228. Studies in the azole series. Part XIV. A new synthesis of purines". Journal of the Chemical Society (Resumed). 0 (0): 1071. doi:10.1039/jr9490001071. ISSN 0368-1769.
- ↑ Li, J (2004). Name Reactions A Collection of Detailed Mechanisms and Synthetic Applications, Fifth edition. Springer International Publishing.
- ↑ Hantzsch, A.; Weber, J. H. (July 1887). "Ueber Verbindungen des Thiazols (Pyridins der Thiophenreihe)". Berichte der deutschen chemischen Gesellschaft. 20 (2): 3118–3132. doi:10.1002/cber.188702002200. ISSN 0365-9496.
- 1 2 Scott, David A.; Aquila, Brian M.; Bebernitz, Geraldine A.; Cook, Donald J.; Dakin, Les A.; Deegan, Tracy L.; Hattersley, Maureen M.; Ioannidis, Stephanos; Lyne, Paul D. (2008). "Pyridyl and thiazolyl bisamide CSF-1R inhibitors for the treatment of cancer". Bioorganic & Medicinal Chemistry Letters. 18 (17): 4794–4797. doi:10.1016/j.bmcl.2008.07.093. ISSN 0960-894X.
- 1 2 Ayati, Adile; Emami, Saeed; Asadipour, Ali; Shafiee, Abbas; Foroumadi, Alireza (2015). "Recent applications of 1,3-thiazole core structure in the identification of new lead compounds and drug discovery". European Journal of Medicinal Chemistry. 97: 699–718. doi:10.1016/j.ejmech.2015.04.015. ISSN 0223-5234.