Chiraphos
 | |||
| |||
| Names | |||
|---|---|---|---|
| Other names
* (2S,3S)-(–)-Bis(diphenylphosphino)butane
| |||
| Identifiers | |||
| |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.161.375 | ||
PubChem CID |
|||
| UNII |
| ||
| |||
| |||
| Properties | |||
| C28H28P2 | |||
| Molar mass | 426.47 g/mol | ||
| Appearance | White powder | ||
| Melting point | 104 to 109 °C (219 to 228 °F; 377 to 382 K) | ||
| Hazards | |||
EU classification (DSD) (outdated) |
Irritant (XI) | ||
| R-phrases (outdated) | R36/37/38 | ||
| S-phrases (outdated) | S26 S37/39 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
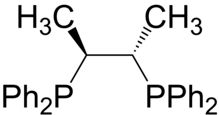
Chiraphos is a chiral diphosphine employed as a ligand in organometallic chemistry. This bidentate ligand chelates metals via the two phosphine groups. Its name is derived from its description — being both chiral and a phosphine. As a C2-symmetric ligand, chiraphos is available in two enantiomeric forms, S,S and R,R, each with C2 symmetry.
Preparation
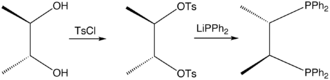
Chiraphos is prepared from S,S or R,R-2,3-butanediol, which are derived from commercially available S,S or R,R-tartaric acid; the technique of using cheaply available enantiopure starting materials is known as chiral pool synthesis. The diol is tosylated and then the ditosylate is treated with lithium diphenylphosphide.[1] The ligand was an important demonstration of how the conformation of the chelate ring can affect asymmetric induction by a metal catalyst. Prior to this work, in most chiral phosphines, e.g., DIPAMP, phosphorus was the stereogenic center.
References
- ↑ Fryzuk, M. D.; Bosnich, B. (1977). "Asymmetric synthesis. Production of optically active amino acids by catalytic hydrogenation". Journal of the American Chemical Society. 99 (19): 6262–6267. doi:10.1021/ja00461a014. PMID 893889.