Carbonate ester

A carbonate ester (organic carbonate or organocarbonate) is an ester of carbonic acid. This functional group consists of a carbonyl group flanked by two alkoxy groups. The general structure of these carbonates is R1O(C=O)OR2 and they are related to esters R1O(C=O)R and ethers R1OR2 and also to the inorganic carbonates.
Monomers of polycarbonate (e.g. Lexan) are linked by carbonate groups. These polycarbonates are used in eyeglass lenses, compact discs, and bulletproof glass. Small carbonate esters like dimethyl carbonate, and ethylene and propylene carbonate are used as solvents. Dimethyl carbonate is also a mild methylating agent.
Types
Carbonate esters can be divided into three categories by their structures. The first and general case is a carbonate group with two simple, identical substituents; depending on whether the substituents are aliphatic or aromatic, they are called dialkyl or diaryl carbonates, respectively. The simplest members of these classes are dimethyl carbonate and diphenyl carbonate. Alternatively, the carbonate groups can be linked by a 2- or 3-carbon bridge, forming cyclic compounds such as ethylene carbonate and trimethylene carbonate. The bridging compound can also have substituents, e.g. CH3 for propylene carbonate. Instead of terminal alkyl or aryl groups, two carbonate groups can be linked by an aliphatic or aromatic bifunctional group. For example, poly(propylene carbonate) and poly(bisphenol A carbonate) (Lexan).
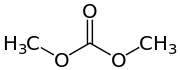 Dimethyl carbonate, a representative acyclic carbonate ester
Dimethyl carbonate, a representative acyclic carbonate ester Diphenyl carbonate, another representative acyclic carbonate ester
Diphenyl carbonate, another representative acyclic carbonate ester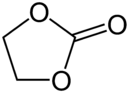 Ethylene carbonate, a cyclic carbonate ester
Ethylene carbonate, a cyclic carbonate ester Trimethylene carbonate, another cyclic carbonate ester
Trimethylene carbonate, another cyclic carbonate ester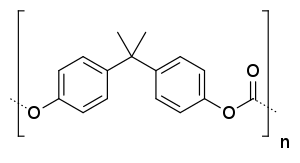 Poly(bisphenol A carbonate), a commercially important plastic (Lexan)
Poly(bisphenol A carbonate), a commercially important plastic (Lexan)
Preparation
There are two main industrial ways of preparing carbonate esters: the reaction of an alcohol (or phenol) with phosgene (phosgenation), and the reaction of an alcohol with carbon monoxide and an oxidizer (oxidative carbonylation). Other carbonate esters may subsequently be prepared by transesterification.[1][2]
Phosgenation
Alcohols react with phosgene to yield carbonate esters according to the following reaction:
- 2 ROH + COCl2 → ROCO2R + 2 HCl
Phenols react similarly. Polycarbonate derived from bisphenol A is produced in this manner. This process is high yielding. However, toxic phosgene is used, and stoichiometric quantities of base (e.g. pyridine) are required to neutralize the hydrogen chloride that is cogenerated.[1][2] Chloroformate esters are intermediates in this process. Rather than reacting with additional alcohol, they may disproportionate to give the desired carbonate diesters and one equivalent of phosgene:[2]
- PhOH + COCl2 → PhOCOCl + HCl
- 2 PhOCOCl → PhOCO2Ph + COCl2
Oxidative carbonylation
With an appropriate catalyst, alcohols can react with carbon monoxide and an oxidant (e.g. oxygen gas). The method is mainly applied to diphenylcarbonate from phenol (PhOH):[1][2]
- 4 PhOH + 2 CO + O2 → 2 (PhO)2CO + 2 H2O
Most catalysts are based on palladium, but none can compete with phosgene routes.[3] In the case of dimethylcarbonylate, this oxidative reaction has been claimed to be competitive with routes based on phosgene.[4]
Reaction of carbon dioxide with epoxides
The reaction of carbon dioxide with epoxides is a general route to the preparation of cyclic 5-membered carbonates. Annual production of cyclic carbonates was estimated at 100,000 tonnes per year in 2010.[5] Industrially, ethylene and propylene oxides readily react with carbon dioxide to give ethylene and propylene carbonates (with an appropriate catalyst).[1][2] For example:
- C2H4O + CO2 → C2H4O2CO
Catalysts for this reaction have been reviewed, as have non-epoxide routes to these cyclic carbonates.[5]
Carbonate transesterification
Once the initial carbonate has been produced, it may be converted to other carbonates by transesterification. A more nucleophilic alcohol will displace a less nucleophilic alcohol. In other words, aliphatic alcohols will displace phenols from aryl carbonates. If the departing alcohol is more volatile, the equilibrium may be driven by distilling that off.[1][2]
Experimental routes
From carbon dioxide and alcohols
In principle carbonate esters can be prepared the direct condensation of methanol and carbon dioxide.[6][7] The reaction is thermodynamically unfavorable, due to the buildup of water byproduct.[8] A selective membrane can be used to separate the water from the reaction mixture and increase the yield.[9][10]
From urea with alcohols
Dimethyl carbonate can be made from the reaction of methanol with urea. Ammonia that is produced can be recycled. Effectively ammonia serves as a catalyst for the synthesis of dimethyl carbonate. The byproducts are methyl- and N-methylcarbamate (the latter from the reaction between dimethyl carbonate and methyl carbamate). The yield of dimethyl carbonate is only 30%, so unless the byproducts are recycled or commercially realized this is not an economic method.[11]
Organic synthesis
Laboratory methods for the synthesis of carbonate ester in the laboratory are from the corresponding diols, or by reaction of an epoxide with carbon dioxide catalysed by a zinc halide.[12]
Use as solvents
A large number of organic carbonates are used as solvents.[13] They are classified as polar solvents and have a wide liquid temperature range. One example is propylene carbonate with melting point −55 °C and boiling point 240 °C. Other advantages are low ecotoxicity and good biodegradability. Many industrial production pathways for carbonates are not green because they rely on phosgene or propylene oxide.[14]
Use in batteries
Organic carbonates are used as a solvent in lithium batteries; due to their high polarity they can dissolve lithium salts. The problem of high viscosity is circumvented by using carbonate mixtures for example mixtures of dimethyl carbonate, diethyl carbonate and dimethoxy ethane.
References
- 1 2 3 4 5 Shaikh, Abbas-Alli G.; Swaminathan Sivaram (1996). "Organic Carbonates". Chemical Reviews. 96 (3): 951–976. doi:10.1021/cr950067i. PMID 11848777.
- 1 2 3 4 5 6 Hans-Josef Buysch, "Carbonic Esters", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a05_197
- ↑ Grigorii L. Soloveichik1 (2016). "Oxidative Carbonylation: Diphenyl Carbonate". In Shannon S. Stahl, andPaul L. Alsters. Title Liquid Phase Aerobic Oxidation Catalysis: Industrial Applications and Academic Perspectives: Industrial Applications and Academic Perspectives. Wiley-VCH. doi:10.1002/9783527690121.ch12.
- ↑ Shaikh, Abbas-Alli G.; Sivaram, Swaminathan (1996-01-01). "Organic Carbonates". Chemical Reviews. 96 (3): 951–976. doi:10.1021/cr950067i. ISSN 0009-2665.
- 1 2 North, Michael; Pasquale, Riccardo; Young, Carl (2010). "Synthesis of cyclic carbonates from epoxides and CO2". Green Chem. 12 (9): 1514. doi:10.1039/c0gc00065e.
- ↑ http://www.mdpi.com/1422-0067/11/4/1343
- ↑ Bian, Jun. "Highly effective synthesis of dimethyl carbonate from methanol and carbon dioxide using a novel copper–nickel/graphite bimetallic nanocomposite catalyst". Chemical Engineering Journal. 147: 287–296. doi:10.1016/j.cej.2008.11.006.
- ↑ Zhang, Zhi-Fang. "Synthesis of Dimethyl Carbonate from Carbon Dioxide and Methanol over CexZr1-xO2and [EMIM]Br/Ce0.5Zr0.5O2". Industrial & Engineering Chemistry Research. 50: 1981–1988. doi:10.1021/ie102017j.
- ↑ Li, Chuan-Feng. "Study on application of membrane reactor in direct synthesis DMC from CO2 and CH3OH over Cu–KF/MgSiO catalyst". Catalysis Today. 82: 83–90. doi:10.1016/S0920-5861(03)00205-0.
- ↑ http://alexandria.tue.nl/extra1/afstversl/st/vermerris2005.pdf
- ↑ "Archived copy" (PDF). Archived from the original (PDF) on 2013-10-05. Retrieved 2013-10-04.
- ↑ Zinc(II)-pyridine-2-carboxylate / 1-methyl-imidazole: a binary catalytic system for in the synthesis of cyclic carbonates from carbon dioxide and epoxides Arkivoc 2007 (iii) 151-163 (EA-2262DP) Thomas A. Zevaco, Annette Janssen, and Eckhard Dinjus Link
- ↑ Schäffner, B.; Schäffner, F.; Verevkin, S. P.; Börner, A. (2010). "Organic Carbonates as Solvents in Synthesis and Catalysis". Chemical Reviews. 110 (8): 4554–4581. doi:10.1021/cr900393d. PMID 20345182.
- ↑ Sibiya, Mike Sbonelo. Catalytic transformation of propylene carbonate into dimethyl carbonate and propylene glycol