Carbadox
 | |
| Names | |
|---|---|
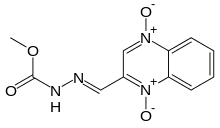
| IUPAC name
methyl (2E)-2-[(1,4-dioxidoquinoxalin-2-yl) | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.027.163 |
| EC Number | 229-879-0 |
PubChem CID |
|
| RTECS number | FE2779000 |
| UNII | |
| |
| |
| Properties | |
| C11H10N4O4 | |
| Molar mass | 262.22 g/mol |
| Appearance | Yellow crystals |
| Density | 1.44 g/cm3 |
| Melting point | 239.5 °C |
| Insoluble | |
| Hazards | |
EU classification (DSD) (outdated) |
F, T |
| R/S statement (outdated) | R: R45, R11, R22 S: S53, S45 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Carbadox is a veterinary drug that combats bacterial infection in swine, particularly swine dysentery. In early 2004, it was banned by the Canadian government as a livestock feed additive and for human consumption, since it is carcinogenic and induces birth defects, as was shown in animal experiments.[1] The European Union also forbids the use of carbadox at any level.[2] It is approved in the United States for use in swine for up to 42 days before slaughter,[3] but in 2016, the United States Food and Drug Administration moved to ban its use in pork, citing a potential cancer risk to humans.[4] Australia forbids the use of carbadox in food producing animals.[5]
References
- ↑ Maximum Residue Limits, Health Canada, retrieved 2010-07-27
- ↑ Ungemach, Fritz R. "WHO Food Additives Series: 51 CARBADOX (addendum)". WHO Food Additives Series. INCHEM. Retrieved 23 Mar 2015.
- ↑ "21CFR 558.115". Code of Federal Regulations. FDA. 1 Apr 2014. Retrieved 23 Mar 2015.
- ↑ Fox, Maggie. "FDA Moves to Ban Cancer-Causing Pork Antibiotic". NBC News. Retrieved 9 Apr 2016.
- ↑ Substances Not Permitted for use on Food-Producing Animals in Australia, Australian Pesticides and Veterinary Medicines Authority, retrieved 2010-08-31
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.