Camostat
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
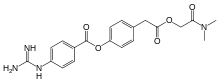
| Formula | C20H22N4O5 |
| Molar mass | 398.41 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Camostat (INN) or FOY-305 is a serine protease inhibitor. Serine protease enzymes have a variety of functions in the body, and so camostat has a diverse range of uses. It is used in the treatment of some forms of cancer and is also effective against some viral infections, as well as inhibiting fibrosis in liver or kidney disease or pancreatitis.[1][2][3][4][5][6]
References
- ↑ Okuno M, Kojima S, Akita K, Matsushima-Nishiwaki R, Adachi S, Sano T, Takano Y, Takai K, Obora A, Yasuda I, Shiratori Y, Okano Y, Shimada J, Suzuki Y, Muto Y, Moriwaki Y. Retinoids in liver fibrosis and cancer. Front Biosci. 2002 Jan 1;7:d204-18. PMID 11779708
- ↑ Motoo Y. Antiproteases in the treatment of chronic pancreatitis. JOP. 2007 Jul 9;8(4 Suppl):533-7. PMID 17625311
- ↑ Hsieh HP, Hsu JT. Strategies of development of antiviral agents directed against influenza virus replication. Curr Pharm Des. 2007;13(34):3531-42. PMID 18220789
- ↑ Kitamura K, Tomita K. Proteolytic activation of the epithelial sodium channel and therapeutic application of a serine protease inhibitor for the treatment of salt-sensitive hypertension. Clin Exp Nephrol. 2012 Feb;16(1):44-8. doi: 10.1007/s10157-011-0506-1 PMID 22038264
- ↑ Zhou Y, Vedantham P, Lu K, Agudelo J, Carrion R Jr, Nunneley JW, Barnard D, Pöhlmann S, McKerrow JH, Renslo AR, Simmons G. Protease inhibitors targeting coronavirus and filovirus entry. Antiviral Res. 2015 Apr;116:76-84. doi: 10.1016/j.antiviral.2015.01.011 PMID 25666761
- ↑ Ueda M, Uchimura K, Narita Y, Miyasato Y, Mizumoto T, Morinaga J, Hayata M, Kakizoe Y, Adachi M, Miyoshi T, Shiraishi N, Kadowaki D, Sakai Y, Mukoyama M, Kitamura K. The serine protease inhibitor camostat mesilate attenuates the progression of chronic kidney disease through its antioxidant effects. Nephron. 2015;129(3):223-32. doi: 10.1159/000375308 PMID 25766432
- Kunze H, Bohn E (May 1983). "Effects of the serine protease inhibitors FOY and FOY 305 on phospholipase A1 (EC 3.1.1.32) activity in rat - liver lysosomes". Pharmacol Res Commun. 15 (5): 451–9. doi:10.1016/S0031-6989(83)80065-4. PMID 6412250.
- Göke B, Stöckmann F, Müller R, Lankisch PG, Creutzfeldt W (1984). "Effect of a specific serine protease inhibitor on the rat pancreas: systemic administration of camostate and exocrine pancreatic secretion". Digestion. 30 (3): 171–8. doi:10.1159/000199102. PMID 6209186.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.