CPN1
Carboxypeptidase N catalytic chain is an enzyme that in humans is encoded by the CPN1 gene.[5][6][7]
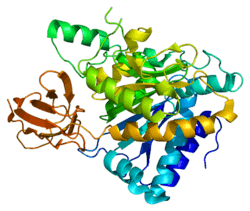
Carboxypeptidase N is a plasma metallo-protease that cleaves basic amino acids from the C terminal of peptides and proteins. The enzyme is important in the regulation of peptides like kinins and anaphylatoxins, and has also been known as kininase-1 and anaphylatoxin inactivator. This enzyme is a tetramer composed of two identical regulatory subunits and two identical catalytic subunits; this gene encodes the catalytic subunit. Mutations in this gene can be associated with angioedema or chronic urticaria resulting from carboxypeptidase N deficiency.[7]
In melanocytic cells CPN1 gene expression may be regulated by MITF.[8]
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000120054 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000025196 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Riley DA, Tan F, Miletich DJ, Skidgel RA (Apr 1999). "Chromosomal localization of the genes for human carboxypeptidase D (CPD) and the active 50-kilodalton subunit of human carboxypeptidase N (CPN1)". Genomics. 50 (1): 105–8. doi:10.1006/geno.1998.5295. PMID 9628828.
- ↑ Gebhard W, Schube M, Eulitz M (Mar 1989). "cDNA cloning and complete primary structure of the small, active subunit of human carboxypeptidase N (kininase 1)". Eur J Biochem. 178 (3): 603–7. doi:10.1111/j.1432-1033.1989.tb14488.x. PMID 2912725.
- 1 2 "Entrez Gene: CPN1 carboxypeptidase N, polypeptide 1".
- ↑ Hoek KS, Schlegel NC, Eichhoff OM, et al. (2008). "Novel MITF targets identified using a two-step DNA microarray strategy". Pigment Cell Melanoma Res. 21 (6): 665–76. doi:10.1111/j.1755-148X.2008.00505.x. PMID 19067971.
External links
- Human CPN1 genome location and CPN1 gene details page in the UCSC Genome Browser.
Further reading
- Matthews KW, Mueller-Ortiz SL, Wetsel RA (2004). "Carboxypeptidase N: a pleiotropic regulator of inflammation". Mol. Immunol. 40 (11): 785–93. doi:10.1016/j.molimm.2003.10.002. PMID 14687935.
- Gebhard W, Schube M, Eulitz M (1990). "cDNA cloning of kininase 1". Adv. Exp. Med. Biol. 247B: 261–4. doi:10.1007/978-1-4615-9546-5_43. PMID 2610070.
- Skidgel RA, Bennett CD, Schilling JW, et al. (1988). "Amino acid sequence of the N-terminus and selected tryptic peptides of the active subunit of human plasma carboxypeptidase N: comparison with other carboxypeptidases". Biochem. Biophys. Res. Commun. 154 (3): 1323–9. doi:10.1016/0006-291X(88)90284-7. PMID 3408501.
- Hendriks D, Vingron M, Vriend G, et al. (1994). "On the specificity of carboxypeptidase N, a comparative study". Biol. Chem. Hoppe-Seyler. 374 (9): 843–9. doi:10.1515/bchm3.1993.374.7-12.843. PMID 8267877.
- Sato T, Miwa T, Akatsu H, et al. (2000). "Pro-carboxypeptidase R is an acute phase protein in the mouse, whereas carboxypeptidase N is not". J. Immunol. 165 (2): 1053–8. doi:10.4049/jimmunol.165.2.1053. PMID 10878383.
- Campbell WD, Lazoura E, Okada N, Okada H (2002). "Inactivation of C3a and C5a octapeptides by carboxypeptidase R and carboxypeptidase N.". Microbiol. Immunol. 46 (2): 131–4. doi:10.1111/j.1348-0421.2002.tb02669.x. PMID 11939578.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Cao H, Hegele RA (2003). "DNA polymorphism and mutations in CPN1, including the genomic basis of carboxypeptidase N deficiency". J. Hum. Genet. 48 (1): 20–2. doi:10.1007/s100380300003. PMID 12560874.
- Shimomura Y, Kawamura T, Komura H, et al. (2003). "Modulation of procarboxypeptidase R (ProCPR) activation by complementary peptides to thrombomodulin". Microbiol. Immunol. 47 (3): 241–5. doi:10.1111/j.1348-0421.2003.tb03383.x. PMID 12725295.
- Gerhard DS, Wagner L, Feingold EA, et al. (2004). "The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334.
- Davis DA, Singer KE, De La Luz Sierra M, et al. (2005). "Identification of carboxypeptidase N as an enzyme responsible for C-terminal cleavage of stromal cell-derived factor-1alpha in the circulation". Blood. 105 (12): 4561–8. doi:10.1182/blood-2004-12-4618. PMC 1895000. PMID 15718415.
- Rual JF, Venkatesan K, Hao T, et al. (2005). "Towards a proteome-scale map of the human protein-protein interaction network". Nature. 437 (7062): 1173–8. doi:10.1038/nature04209. PMID 16189514.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.