Butler–Volmer equation
The Butler–Volmer equation (named after John Alfred Valentine Butler[1] and Max Volmer), also known as Erdey-Grúz–Volmer equation, is one of the most fundamental relationships in electrochemical kinetics. It describes how the electrical current on an electrode depends on the electrode potential, considering that both a cathodic and an anodic reaction occur on the same electrode:[2]
or in a more compact form:
where:
- : electrode current density, A/m2 (defined as i = I/A)
- : exchange current density, A/m2
- : electrode potential, V
- : equilibrium potential, V
- : absolute temperature, K
- : number of electrons involved in the electrode reaction
- : Faraday constant
- : universal gas constant
- : so-called cathodic charge transfer coefficient, dimensionless
- : so-called anodic charge transfer coefficient, dimensionless
- : activation overpotential (defined as ).
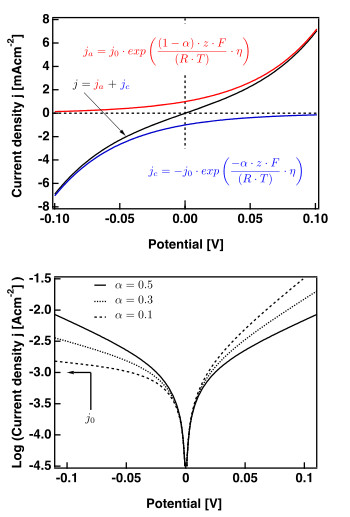
The right hand figure shows plots valid for .
Mass-transfer control
The previous form of Butler–Volmer equation is valid when the electrode reaction is controlled by electrical charge transfer at the electrode (and not by the mass transfer to or from the electrode surface from or to the bulk electrolyte). Nevertheless, the utility of the Butler–Volmer equation in electrochemistry is wide, and it is often considered to be "central in the phenomenological electrode kinetics".[3]
In the region of the limiting current, when the electrode process is mass-transfer controlled, the value of the current density is:
where:
- Deff is the effective diffusion coefficient (taking tortuosity into account, if any);
- δ is the diffusion layer thickness;
- C* is the concentration of the electroactive (limiting) species in the bulk of the electrolyte.
The more general form of the Butler–Volmer equation, applicable to the mass transfer-influenced conditions, can be written as:[4]
where:
- i is the current density, A/m2,
- Co and Cr refer to the concentration of the species to be oxidized and to be reduced, respectively,
- C(0,t) is the time-dependent concentration at the distance zero from the surface.
The above form simplifies to the conventional one (shown at the top of the article) when the concentration of the electroactive species at the surface is equal to that in the bulk.
The limiting cases
There are two limiting cases of the Butler–Volmer equation:
- the low overpotential region (called "polarization resistance", i.e., when E ≈ Eeq), where the Butler–Volmer equation simplifies to:
- ;
- the high overpotential region, where the Butler–Volmer equation simplifies to the Tafel equation:
- for a cathodic reaction, when E << Eeq, or
- for an anodic reaction, when E >> Eeq
where and are constants (for a given reaction and temperature) and are called the Tafel equation constants. The theoretical values of the Tafel equation constants are different for the cathodic and anodic processes.
See also
References
- ↑ Mayneord, W. V. (1979). "John Alfred Valentine Butler, 14 February 1899 - 16 July 1977". Biographical Memoirs of Fellows of the Royal Society. 25: 144–178. doi:10.1098/rsbm.1979.0004.
- ↑ Adler, S.B. "Chapter 11: Sources of cell and electrode polarisation losses in SOFCs". In Kendall, Kevin; Kendall, Michaela. High-Temperature Solid Oxide Fuel Cells for the 21st Century (2nd ed.). Academic Press. doi:10.1016/C2011-0-09278-5. ISBN 9780124104532.
- ↑ J. O'M. Bockris, A.K.N.Reddy, and M. Gamboa-Aldeco, "Modern Electrochemistry 2A. Fundamentals of Electrodics.", Second Edition, Kluwer Academic/Plenum Publishers, p.1083, 2000.
- ↑ Allen Bard and Larry Faulkner, "Electrochemical Methods. Fundamentals and Applications". 2nd edition, John Wiley and Sons, Inc., 2001.