Borane carbonyl
 | |
| Names | |
|---|---|
| Other names
Carbonyltrihydroboron | |
| Identifiers | |
| ChemSpider | |
| Properties | |
| CH3BO | |
| Molar mass | 41.84 g·mol−1 |
| Appearance | colorless gas |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
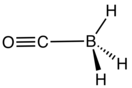
Borane carbonyl is the inorganic compound with the formula H3BCO. This colorless gas is the adduct of borane and carbon monoxide. It is usually prepared by combining borane-ether complexes and CO. The compound is mainly of theoretical and pedagogical interest.[1] It reacts with aqueous base to give Boranocarbonate H3BCO22−.[2] Bond distances are B-C, 1.529; C-O, 1.140; 1.194 Å. The H-B-H angle is 113.7°. The CO vibrational band is at 2165 cm−1, 22 cm−1 higher than that of free CO.[3]
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 165. ISBN 0-08-037941-9.
- ↑ Alberto, R.; Ortner, K.; Wheatley, N.; Schibli, R.; Schubiger, A. P. (2001). "Synthesis and Properties of Boranocarbonate: A Convenient in Situ CO Source for the Aqueous Preparation of [99mTc(OH2)3(CO)3]+". J. Am. Chem. Soc. 123: 3135–3136. doi:10.1021/ja003932b.
- ↑ Jacobsen, H.; Berke, H.; Doering, S.; Kehr, G.; Erker, G.; Froehlich, R.; Meyer, O. (1999). "Lewis Acid Properties of Tris(pentafluorophenyl)borane. Structure and Bonding in L-B(C6F5)3 Complexes". Organometallics. 18: 1724–1735. doi:10.1021/OM981033E.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.