Bicoid (gene)
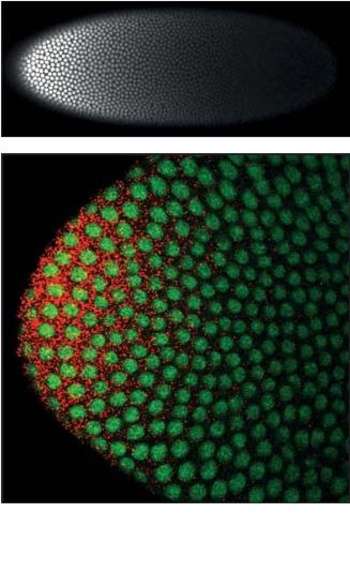
Bicoid is a maternal effect gene whose protein concentration gradient patterns the anterior-posterior (A-P) axis during Drosophila embryogenesis. Bicoid was also the first protein demonstrated to act as a morphogen.[2] Although Bicoid is important for Drosophila development, it is absent from most other insects where its lack is compensated by other genes.[3][4]
Role of Bicoid in axial patterning
bicoid mRNA is actively localized to the anterior of the fruit fly egg during oogenesis[5] along microtubules[6] by the motor protein dynein[7] and retained there through association with cortical actin.[8] Translation of bicoid is regulated by its 3' UTR and begins after egg deposition: diffusion and convection within the syncytium produce an exponential gradient of Bicoid protein[2][9] within roughly one hour, after which Bicoid nuclear concentrations remain approximately constant through cellularization.[10] An alternative model proposes the formation of a bicoid mRNA gradient in the embryo along cortical microtubules which then serves as template for translation of the Bicoid protein to form the Bicoid protein gradient.[11][12][13] Bicoid protein represses the translation of caudal mRNA and enhances the transcription of anterior gap genes including hunchback, orthodenticle, and buttonhead.
Structure and function of Bicoid
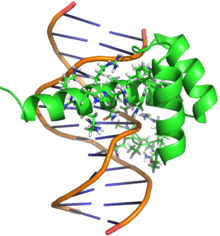
Bicoid is one of few proteins which binds both RNA and DNA targets using its homeodomain to regulate their transcription and translation, respectively. The nucleic acid-binding homeodomain of Bicoid has been solved by NMR.[14] Bicoid contains an arginine-rich motif (part of the helix shown axially in this image) that is similar to the one found in the HIV protein REV is essential for its nucleic acid binding.[15]
Bicoid protein gradient formation is one of the earliest steps in fruit fly embryo A-P patterning: the proper spatial expression of downstream genes relies on the robustness of this gradient to common variations between embryos, including in the number of maternally-deposited bicoid mRNAs and in egg size. Comparative phylogenetic[16] and experimental evolution[17] studies suggest an inherent mechanism for robust generation of a scaled Bicoid protein gradient. Mechanisms that have been proposed to effect this scaling include non-linear degradation of Bicoid,[18] nuclear retention as a size-dependent regulator of Bicoid protein's effective diffusion coefficient,[9][19] and scaling of cytoplasmic streaming.[9]
See also
References
- ↑ Little, S. C.; Tkačik, G. P.; Kneeland, T. B.; Wieschaus, E. F.; Gregor, T. (2011). "The Formation of the Bicoid Morphogen Gradient Requires Protein Movement from Anteriorly Localized mRNA". PLoS Biology. 9 (3): e1000596. doi:10.1371/journal.pbio.1000596. PMC 3046954. PMID 21390295.
- 1 2 Driever, W.; Nüsslein-Volhard, C. (1988). "The bicoid protein determines position in the Drosophila embryo in a concentration-dependent manner". Cell. 54 (1): 95–104. doi:10.1016/0092-8674(88)90183-3. PMID 3383245.
- ↑ Chouard, Tanguy (2008-11-20). "Darwin 200: Beneath the surface". Nature. 456 (7220): 300–303. doi:10.1038/456300a. ISSN 1476-4687. PMID 19020592.
- ↑ Schröder, Reinhard (10 April 2003). "The genes orthodenticle and hunchback substitute for bicoid in the beetle Tribolium". Nature. 422 (6932): 621–625. doi:10.1038/nature01536. ISSN 0028-0836. PMID 12687002.
- ↑ St. Johnston D, Driever W, Berleth T, Richstein S, Nusslein-Volhard C (1989). Multiple steps in the localization of bicoid RNA to the anterior pole of the Drosophila oocyte. Development 107 Suppl: 13–19.
- ↑ Pokrywka, N. J.; Stephenson, E. C. (1991). "Microtubules mediate the localization of bicoid RNA during Drosophila oogenesis". Development. 113 (1): 55–66. PMID 1684934.
- ↑ Weil, T. T.; Forrest, K. M.; Gavis, E. R. (2006). "Localization of bicoid mRNA in Late Oocytes is Maintained by Continual Active Transport". Developmental Cell. 11 (2): 251–262. doi:10.1016/j.devcel.2006.06.006. PMID 16890164.
- ↑ Weil, T. T.; Parton, R.; Davis, I.; Gavis, E. R. (2008). "Changes in bicoid mRNA Anchoring Highlight Conserved Mechanisms during the Oocyte-to-Embryo Transition". Current Biology. 18 (14): 1055–1061. doi:10.1016/j.cub.2008.06.046. PMC 2581475. PMID 18639459.
- 1 2 3 Hecht, I.; Rappel, W. -J.; Levine, H. (2009). "Determining the scale of the Bicoid morphogen gradient". Proceedings of the National Academy of Sciences. 106 (6): 1710–1715. doi:10.1073/pnas.0807655106. PMC 2644102. PMID 19190186.
- ↑ Gregor, T.; Wieschaus, E. F.; McGregor, A. P.; Bialek, W.; Tank, D. W. (2007). "Stability and Nuclear Dynamics of the Bicoid Morphogen Gradient". Cell. 130 (1): 141–152. doi:10.1016/j.cell.2007.05.026. PMC 2253672. PMID 17632061.
- ↑ Frigerio, G; Burri, M; Bopp, D; Baumgartner, S; Noll, M (Dec 1986). "Structure of the segmentation gene paired and the Drosophila PRD gene set as part of a gene network". Cell. 47 (5): 735–46. doi:10.1016/0092-8674(86)90516-7. PMID 2877746.
- ↑ Spirov, A; Fahmy, K; Schneider, M; Frei, E; Noll, M; Baumgartner, S (Feb 2009). "Formation of the bicoid morphogen gradient: an mRNA gradient dictates the protein gradient". Development. 136 (4): 605–14. doi:10.1242/dev.031195. PMC 2685955. PMID 19168676.
- ↑ Fahmy, Khalid; Akber, Mira; Cai, Xiaoli; Koul, Aabid; Hayder, Awais; Baumgartner, Stefan (2014). "ΑTubulin 67C and Ncd Are Essential for Establishing a Cortical Microtubular Network and Formation of the Bicoid mRNA Gradient in Drosophila". PLOS ONE. 9 (11): e112053. doi:10.1371/journal.pone.0112053. PMC 4229129. PMID 25390693.
- ↑ Baird-Titus, J. M.; Clark-Baldwin, K.; Dave, V.; Caperelli, C. A.; Ma, J.; Rance, M. (2006). "The Solution Structure of the Native K50 Bicoid Homeodomain Bound to the Consensus TAATCC DNA-binding Site". Journal of Molecular Biology. 356 (5): 1137–1151. doi:10.1016/j.jmb.2005.12.007. PMID 16406070.
- ↑ Niessing, D.; Driever, W.; Sprenger, F.; Taubert, H.; Jäckle, H.; Rivera-Pomar, R. (2000). "Homeodomain Position 54 Specifies Transcriptional versus Translational Control by Bicoid". Molecular Cell. 5 (2): 395–401. doi:10.1016/S1097-2765(00)80434-7. PMID 10882080.
- ↑ Gregor, T.; McGregor, A. P.; Wieschaus, E. F. (2008). "Shape and function of the Bicoid morphogen gradient in dipteran species with different sized embryos". Developmental Biology. 316 (2): 350–358. doi:10.1016/j.ydbio.2008.01.039. PMC 2441567. PMID 18328473.
- ↑ Cheung, D.; Miles, C.; Kreitman, M.; Ma, J. (2013). "Adaptation of the length scale and amplitude of the Bicoid gradient profile to achieve robust patterning in abnormally large Drosophila melanogaster embryos". Development. 141 (1): 124–35. doi:10.1242/dev.098640. PMC 3865754. PMID 24284208.
- ↑ Eldar, A.; Rosin, D.; Shilo, B. Z.; Barkai, N. (2003). "Self-Enhanced Ligand Degradation Underlies Robustness of Morphogen Gradients". Developmental Cell. 5 (4): 635–46. doi:10.1016/S1534-5807(03)00292-2. PMID 14536064.
- ↑ Grimm, O.; Wieschaus, E. (2010). "The Bicoid gradient is shaped independently of nuclei". Development. 137 (17): 2857–2862. doi:10.1242/dev.052589. PMC 2938918. PMID 20699297.