Betrixaban
 | |
| Clinical data | |
|---|---|
| Trade names | Bevyxxa |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard |
100.207.746 |
| Chemical and physical data | |
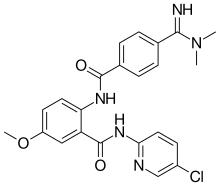
| Formula | C23H22ClN5O3 |
| Molar mass | 451.905 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Betrixaban (INN; trade name Bevyxxa; development code PRT-054,021) is an anticoagulant drug which acts as a direct factor Xa inhibitor.[1] It is potent, orally active and highly selective for factor Xa, being selected from a group of similar compounds for its low hERG affinity.[2] Betrixaban has undergone human clinical trials for prevention of embolism after knee surgery,[3] and prevention of stroke following atrial fibrillation,[4] with promising results.[5] Betrixaban is currently being studied in a human clinical trial for extended duration thromboprophylaxis to prevent venous thromboembolism in acute medically ill patients.[6] Joint development with Portola was discontinued in 2011 by Merck.[7] Betrixaban is now being developed by Portola Pharmaceuticals; the drug received FDA approval on June 23rd, 2017.[8]
See also
References
- ↑ Eriksson BI, Quinlan DJ, Weitz JI (2009). "Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor xa inhibitors in development". Clinical Pharmacokinetics. 48 (1): 1–22. doi:10.2165/0003088-200948010-00001. PMID 19071881.
- ↑ Zhang P, Huang W, Wang L, Bao L, Jia ZJ, Bauer SM, Goldman EA, Probst GD, Song Y, Su T, Fan J, Wu Y, Li W, Woolfrey J, Sinha U, Wong PW, Edwards ST, Arfsten AE, Clizbe LA, Kanter J, Pandey A, Park G, Hutchaleelaha A, Lambing JL, Hollenbach SJ, Scarborough RM, Zhu BY (April 2009). "Discovery of betrixaban (PRT054021), N-(5-chloropyridin-2-yl)-2-(4-(N,N-dimethylcarbamimidoyl)benzamido)-5-methoxybenzamide, a highly potent, selective, and orally efficacious factor Xa inhibitor". Bioorganic & Medicinal Chemistry Letters. 19 (8): 2179–85. doi:10.1016/j.bmcl.2009.02.111. PMID 19297154.
- ↑ Turpie AG, Bauer KA, Davidson BL, Fisher WD, Gent M, Huo MH, Sinha U, Gretler DD (January 2009). "A randomized evaluation of betrixaban, an oral factor Xa inhibitor, for prevention of thromboembolic events after total knee replacement (EXPERT)". Thrombosis and Haemostasis. 101 (1): 68–76. doi:10.1160/th08-07-0460. PMID 19132191.
- ↑ Piccini, J. P.; Lopes, R. D.; Mahaffey, K. W. (2010). "Oral factor Xa inhibitors for the prevention of stroke in atrial fibrillation". Current Opinion in Cardiology. 25 (4): 312–20. doi:10.1097/HCO.0b013e32833a524f. PMID 20520539.
- ↑ Sobieraj-Teague, M.; O’donnell, M.; Eikelboom, J. (2009). "New Anticoagulants for Atrial Fibrillation". Seminars in Thrombosis and Hemostasis. 35 (5): 515–24. doi:10.1055/s-0029-1234147. PMID 19739042.
- ↑ Cohen, Alexander T.; Harrington, Robert; Goldhaber, Samuel Z.; Hull, Russell; Gibson, C. Michael; Hernandez, Adrian F.; Kitt, Michael M.; Lorenz, Todd J. (2014-01-01). "The design and rationale for the Acute Medically Ill Venous Thromboembolism Prevention with Extended Duration Betrixaban (APEX) study". American Heart Journal. 167 (3): 335. doi:10.1016/j.ahj.2013.11.006. PMID 24576517.
- ↑ Husten, Harry. "Merck Abandons Development of Factor Xa Inhibitor Betrixaban". CardioBrief. Retrieved 11 April 2014.
- ↑ "FDA approved betrixaban (BEVYXXA, Portola) for the prophylaxis of venous thromboembolism (VTE) in adult patients". Retrieved 28 June 2017.