Beta diversity
In ecology, beta diversity (β-diversity or true beta diversity) is the ratio between regional and local species diversity. The term was introduced by R. H. Whittaker[1] together with the terms alpha diversity (α-diversity) and gamma diversity (γ-diversity). The idea was that the total species diversity in a landscape (γ) is determined by two different things, the mean species diversity at the habitat level (α) and the differentiation among habitats (β). Other formulations for beta diversity include "absolute species turnover", "Whittaker's species turnover" and "proportional species turnover".
Whittaker proposed several ways of quantifying differentiation, and subsequent generations of ecologists have invented more. As a result, there are now many defined types of beta diversity.[2][3] Some use beta diversity to refer to any of several indices related to compositional heterogeneity.[4][5][6] Confusion is avoided by using distinct names for other formulations.[2][3][7][8][9][10]
Beta diversity as a measure of species turnover overemphasizes the role of rare species as the difference in species composition between two sites or communities is likely reflecting the presence and absence of some rare species in the assemblages. Beta diversity can also be a measure of nestedness, which occurs when species assemblages in species-poor sites are a subset of the assemblages in more species-rich sites [11]. Moreover, pairwise beta diversity are inadequate in building all biodiversity partitions (some partitions in a Venn diagram of 3 or more sites cannot be expressed by alpha and beta diversity). Consequently, some macroecological and community patterns cannot be fully expressed by alpha and beta diversity. Due to these two reasons, a new way of measuring species turnover, coined Zeta diversity (ζ-diversity),[12] has been proposed and used to connect all existing incidence-based biodiversity patterns.
Types
True beta diversity
Gamma diversity and alpha diversity can be calculated directly from species inventory data.[2][13] The simplest of Whittaker's original definitions of beta diversity is
β = γ/α
Here gamma diversity is the total species diversity of a landscape and alpha diversity is the mean species diversity per habitat. Because the limits among habitats and landscapes are diffuse and to some degree subjective, it has been proposed that gamma diversity can be quantified for any inventory dataset and that alpha and beta diversity can be quantified whenever the dataset is divided into subunits. Then gamma diversity is the total species diversity in the dataset and alpha diversity the mean species diversity per subunit. Beta diversity quantifies how many subunits there would be if the total species diversity of the dataset and the mean species diversity per subunit remained the same, but the subunits shared no species.[2][7]
Absolute species turnover
Some researchers have preferred to partition gamma diversity into additive rather than multiplicative components.[14][15] Then the beta component of diversity becomes
βA = γ - α
This quantifies how much more species diversity the entire dataset contains than an average subunit within the dataset. This can also be interpreted as the total amount of species turnover among the subunits in the dataset.[2]
When there are two subunits, and presence-absence data are used, this can be calculated with the following equation:
where, S1= the total number of species recorded in the first community, S2= the total number of species recorded in the second community, and c= the number of species common to both communities.
Whittaker's species turnover
If absolute species turnover is divided by alpha diversity, a measure is obtained that quantifies how many times the species composition changes completely among the subunits of the dataset. This measure was proposed by Whittaker,[17] so it has been called Whittaker's species turnover.[2] It is calculated as
βW = (γ - α)/α = γ/α - 1
When there are two subunits, and presence-absence data are used, this equals the one-complement of the Sørensen similarity index.[2][18]
Proportional species turnover
If absolute species turnover is divided by gamma diversity, a measure is obtained that quantifies what proportion of the species diversity in the dataset is not contained in an average subunit.[2] It is calculated as
βP = (γ - α)/γ = 1 - α/γ
When there are two subunits, and presence-absence data are used, this measure as ranged to the interval [0, 1] equals the one-complement of the Jaccard similarity index.[2]
β-diversity patterns
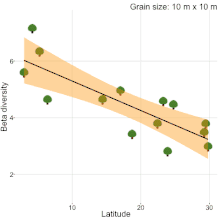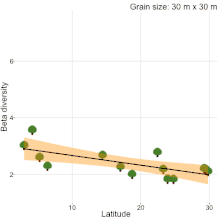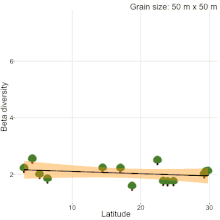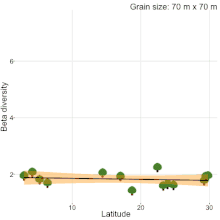
Although, understanding the change in species composition from local to regional scales (β-diversity) is a central theme in ecology and biogeography, studies often reached different conclusions as to the fundamental patterns in β-diversity. For example, niche compression hypothesis predicted higher β-diversity at lower latitudes[20], but, there was never a consensus[19][21][22][23]. Studies comparing natural habitats with human-modified habitats are no different. Kitching et al.[24] sampled moths in primary and logged forests of Danum valley, Borneo to show that β-diversity in primary forests is higher than logged forests. Contrastingly, Berry et al.[25] sampled trees in the same study area to show that β-diversity in logged forests is higher than primary forests. The results of these two studies were completely different from the results of a recent quantitative synthesis[26], which showed that β-diversity in primary forest is similar to β-diversity in all types of human-modified habitats (secondary forests, plantations, pasture and urban). Therefore, there is a clear lack of consensus on β-diversity patterns among studies. Sreekar et al. [19] suggests that most of the inconsistency is due to the differences in grain size and/or spatial extent among studies. They showed that spatial scale changes the relationship between β-diversity and latitude.
See also
References
- ↑ Whittaker, R. H. (1960) Vegetation of the Siskiyou Mountains, Oregon and California. Ecological Monographs, 30, 279–338. doi:10.2307/1943563
- 1 2 3 4 5 6 7 8 9 Tuomisto, H. (2010) A diversity of beta diversities: straightening up a concept gone awry. Part 1. Defining beta diversity as a function of alpha and gamma diversity. Ecography 33: 2–22. doi:10.1111/j.1600-0587.2009.05880.x
- 1 2 Tuomisto, H. (2010) A diversity of beta diversities: straightening up a concept gone awry. Part 2. Quantifying beta diversity and related phenomena. Ecography, 33, 23-45. doi:10.1111/j.1600-0587.2009.06148.x
- ↑ Koleff, P., Gaston, K. J. and Lennon, J. J. (2003) Measuring beta diversity for presence–absence data. Journal of Animal Ecology 72, 367–382. doi: 10.1046/j.1365-2656.2003.00710.x
- ↑ Anderson, M. J. et al. (2011) Navigating the multiple meanings of β diversity: a roadmap for the practicing ecologist. Ecology Letters 14, 19–28. doi:10.1111/j.1461-0248.2010.01552.x
- ↑ Gorelick, R. (2011) Commentary: Do we have a consistent terminology for species diversity? The fallacy of true diversity. Oecologia 167, 885-888. doi:10.1007/s00442-011-2124-8
- 1 2 Tuomisto, H. 2010. A consistent terminology for quantifying species diversity? Yes, it does exist. Oecologia 4: 853–860. doi:10.1007/s00442-010-1812-0
- ↑ Jurasinski, G. and Koch, M. (2011) Commentary: do we have a consistent terminology for species diversity? We are on the way. Oecologia, 167, 893-902. doi:10.1007/s00442-011-2126-6
- ↑ Moreno, C. E: and Rodríguez, P. (2011) Commentary: Do we have a consistent terminology for species diversity? Back to basics and toward a unifying framework. Oecologia, 167, 889-892. doi:10.1007/s00442-011-2125-7
- ↑ Tuomisto, H. (2011) Commentary: do we have a consistent terminology for species diversity? Yes, if we choose to use it. Oecologia, 167, 903-911. doi:10.1007/s00442-011-2128-4
- ↑ Baselga, Andrés (2010-01-01). "Partitioning the turnover and nestedness components of beta diversity". Global Ecology and Biogeography. 19 (1): 134–143. doi:10.1111/j.1466-8238.2009.00490.x. ISSN 1466-8238.
- ↑ Hui, C. & McGeoch, M.A. (2014) Zeta diversity as a concept and metric that unifies incidence-based biodiversity patterns. American Naturalist, 184: 684-694. doi:10.1086/678125
- ↑ Jost L (2006) Entropy and diversity. Oikos 113, 363–375. doi: 10.1111/j.2006.0030-1299.14714.x
- ↑ Lande, R. (1996) Statistics and partitioning of species diversity, and similarity among multiple communities. Oikos, 76, 5-13.
- ↑ Veech, J. A. et al. (2002) The additive partitioning of species diversity: recent revival of an old idea. Oikos, 99, 3-9. doi: 10.1034/j.1600-0706.2002.990101.x
- ↑ James S. Albert; Roberto E. Reis (8 March 2011). Historical Biogeography of Neotropical Freshwater Fishes. University of California Press. p. 308. ISBN 978-0-520-26868-5. Retrieved 28 June 2011.
- ↑ Whittaker, R. H. (1972) Evolution and measurement of species diversity. Taxon, 21, 213-251.doi:10.2307/1218190
- ↑ Sørensen, T.A. (1948) A method of establishing groups of equal amplitude in plant sociology based on similarity of species content, and its application to analyses of the vegetation on Danish commons. Kongelige Danske Videnskabernes Selskabs Biologiske Skrifter, 5, 1–34.
- 1 2 3 Sreekar, R., et al. Spatial scale changes the relationship between beta diversity, species richness and latitude, R. Soc. Open Sci., 5, 181168 (2018).
- ↑ MacArthur, R.H. Patterns of species diversity. Biol. Rev. Camb. Philos. Soc., 40, 510-533 (1965)
- ↑ Koleff, P., Lennon, J.J. and Gaston, K.J. Are there latitudinal gradients in species turnover? Glob. Ecol. Biogeogr., 12, 483-498 (2003).
- ↑ Kraft, N.J.B, et al. Disentangling the drivers of β diversity along latitudinal and elevational gradients. Science, 333, 1755-1758 (2011).
- ↑ Qian, H., Chen, S., Mao, L. and Ouyang, Z. Drivers of β-diversity along latitudinal gradients revisited. Glob. Ecol. Biogeogr. 22, 659-670 (2013).
- ↑ Kitching, R.L., Ashton, L.A., Nakamura, A., Whitaker, T. and Khen C.V. Distance-driven species turnover in Bornean rainforests: homogeneity and heterogeneity in primary and post-logging forests. Ecography 36:675-682 (2013).
- ↑ Berry, N.J., Phillips, O.L., Ong, R.C., Hamer, K.C. Impacts of selective logging on tree diversity across a rainforest landscape: the importance of spatial scale. Landsc. Ecol., 23: 915-929 (2008).
- ↑ Newbold, T., et.al. Global patterns of terrestrial assemblage turnover within and among land uses. Ecography, 39, 1151-1163 (2016).