Barium metaphosphate
| Names | |
|---|---|
| IUPAC name
barium(2+); dioxido(oxo)phosphanium | |
| Identifiers | |
| ECHA InfoCard | 100.033.951 |
PubChem CID |
|
| Properties | |
| Ba(PO3)2 | |
| Molar mass | 295.27 g/mol |
| Appearance | Powder[1] |
| Density | 3.63 g/cm3 |
| Melting point | 1,560[2] °C (2,840 °F; 1,830 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Barium metaphosphate is an inorganic substance with the molecular formula Ba(PO3)2. It is a colourless solid that is insoluble in water, though is soluble in acidic solutions through "slow dissolution"[3]. X-ray crystallography shows that this material is composed of Ba2+ cations attached to a polyphosphate ((PO3−)n) anion.[4] A number of hydrated forms are known which are actually cyclic metaphosphates, Ba2(P4O12)·3.5H2O, Ba3(P3O9)2·6H2O.[5]
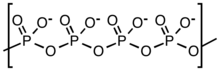
Preparation
Barium metaphosphate can be prepared by the reaction of barium carbonate with metaphosphoric acid:[5]-
- BaCO3 + 2HPO3 → Ba(PO3)2 +CO2 +H2O
or alternatively by the aqueous reaction of barium chloride and sodium metaphosphate:[5]- BaCl2(aq) + 2NaPO3(aq) → Ba(PO3)2 + 2NaCl
Applications
The combination of sodium and barium polyphosphate forms a low-melting glass with a high coefficient of thermal expansion. The melting point of the glass increases with barium content. This glass makes seals with low melting metals like aluminium (melting point 650 °C). Normal borosilicate glasses soften above the melting point of aluminium. This mixture is prepared by heating a mixture of diammonium phosphate, sodium carbonate, and barium carbonate.[6]
References
- ↑ Elements, American. "Barium Metaphosphate". American Elements. Retrieved 17 April 2018.
- ↑ "BARIUM METAPHOSPHATE - 13762-83-9". www.chemicalbook.com. Retrieved 17 April 2018.
- ↑ "Barium Metaphosphate". Chemical Book. 2017. Retrieved November 27, 2017.
- ↑ "Form of barium metaphosphate" Grenier, Jean C.; Martin, Claude M.; Durif, Andre; Tran Qui Duc; Guitel, J. C. Bulletin de la Societe Francaise de Mineralogie et de Cristallographie (1967), vol. 90, 24-31.
- 1 2 3 Ropp, Richard (2012). Encyclopedia of the Alkaline Earth Compounds. Newnes. p. 76. ISBN 0444595538.
- ↑ Wilder Jr., J.A. "Glasses and glass ceramics for sealing to aluminum alloys" Journal of Non-Crystalline Solids Volume 38-39, Issue PART 2, May 1980, Pages 879-884. Hart, Patricia E.; Mesko, Melissa G.; Shelby, James E. "Crystallization and phase equilibrium in the sodium barium metaphosphate system" Journal of Non-Crystalline Solids (2000), 263&264, 305-311. doi:10.1016/S0022-3093(99)00642-0