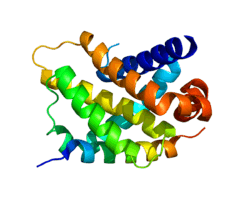
BCL2-related protein A1
Bcl-2-related protein A1 is a protein that in humans is encoded by the BCL2A1 gene.[5][6][7]
Function
This gene encodes a member of the bcl2 protein family. The proteins of this family form hetero- or homodimers and act as anti- and pro-apoptotic regulators that are involved in a wide variety of cellular activities such as embryonic development, homeostasis and tumorigenesis. The protein encoded by this gene is able to reduce the release of pro-apoptotic cytochrome c from mitochondria and block caspase activation. This gene is a direct transcription target of NF-kappa B in response to inflammatory mediators, and has been shown to be up-regulated by different extracellular signals, such as granulocyte-macrophage colony-stimulating factor (GM-CSF), CD40, phorbol ester and inflammatory cytokine TNF and IL-1, which suggests a cytoprotective function that is essential for lymphocyte activation as well as cell survival.[7]
In melanocytic cells BCL2A1 gene expression may be regulated by MITF.[8]
Interactions
BCL2-related protein A1 has been shown to interact with:
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000140379 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000099974 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Savitsky K, Sfez S, Tagle DA, Ziv Y, Sartiel A, Collins FS, Shiloh Y, Rotman G (March 1996). "The complete sequence of the coding region of the ATM gene reveals similarity to cell cycle regulators in different species". Hum Mol Genet. 4 (11): 2025–32. doi:10.1093/hmg/4.11.2025. PMID 8589678.
- ↑ Akatsuka Y, Nishida T, Kondo E, Miyazaki M, Taji H, Iida H, Tsujimura K, Yazaki M, Naoe T, Morishima Y, Kodera Y, Kuzushima K, Takahashi T (June 2003). "Identification of a polymorphic gene, BCL2A1, encoding two novel hematopoietic lineage-specific minor histocompatibility antigens". J Exp Med. 197 (11): 1489–500. doi:10.1084/jem.20021925. PMC 2193899. PMID 12771180.
- 1 2 "Entrez Gene: BCL2A1 BCL2-related protein A1".
- ↑ Hoek KS, Schlegel NC, Eichhoff OM, et al. (2008). "Novel MITF targets identified using a two-step DNA microarray strategy". Pigment Cell Melanoma Res. 21 (6): 665–76. doi:10.1111/j.1755-148X.2008.00505.x. PMID 19067971.
- ↑ Sedlak TW, Oltvai ZN, Yang E, Wang K, Boise LH, Thompson CB, Korsmeyer SJ (Aug 15, 1995). "Multiple Bcl-2 family members demonstrate selective dimerizations with Bax". Proc. Natl. Acad. Sci. U.S.A. 92 (17): 7834–8. doi:10.1073/pnas.92.17.7834. PMC 41240. PMID 7644501.
- ↑ Zhang H, Cowan-Jacob SW, Simonen M, Greenhalf W, Heim J, Meyhack B (2000). "Structural basis of BFL-1 for its interaction with BAX and its anti-apoptotic action in mammalian and yeast cells". J. Biol. Chem. 275 (15): 11092–9. doi:10.1074/jbc.275.15.11092. PMID 10753914.
- ↑ Bae J, Hsu SY, Leo CP, Zell K, Hsueh AJ (2001). "Underphosphorylated BAD interacts with diverse antiapoptotic Bcl-2 family proteins to regulate apoptosis". Apoptosis. 6 (5): 319–30. doi:10.1023/A:1011319901057. PMID 11483855.
- ↑ Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC (2005). "Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function". Mol. Cell. 17 (3): 393–403. doi:10.1016/j.molcel.2004.12.030. PMID 15694340.
External links
- Human BCL2A1 genome location and BCL2A1 gene details page in the UCSC Genome Browser.
Further reading
- Choi SS, Park IC, Yun JW, et al. (1995). "A novel Bcl-2 related gene, Bfl-1, is overexpressed in stomach cancer and preferentially expressed in bone marrow". Oncogene. 11 (9): 1693–8. PMID 7478596.
- Lin EY, Orlofsky A, Berger MS, Prystowsky MB (1993). "Characterization of A1, a novel hemopoietic-specific early-response gene with sequence similarity to bcl-2". J. Immunol. 151 (4): 1979–88. PMID 8345191.
- Karsan A, Yee E, Kaushansky K, Harlan JM (1996). "Cloning of human Bcl-2 homologue: inflammatory cytokines induce human A1 in cultured endothelial cells". Blood. 87 (8): 3089–96. PMID 8605321.
- Karsan A, Yee E, Harlan JM (1996). "Endothelial cell death induced by tumor necrosis factor-alpha is inhibited by the Bcl-2 family member, A1". J. Biol. Chem. 271 (44): 27201–4. doi:10.1074/jbc.271.44.27201. PMID 8910286.
- Carrió R, López-Hoyos M, Jimeno J, et al. (1997). "A1 demonstrates restricted tissue distribution during embryonic development and functions to protect against cell death". Am. J. Pathol. 149 (6): 2133–42. PMC 1865360. PMID 8952545.
- Kenny JJ, Knobloch TJ, Augustus M, et al. (1997). "GRS, a novel member of the Bcl-2 gene family, is highly expressed in multiple cancer cell lines and in normal leukocytes". Oncogene. 14 (8): 997–1001. doi:10.1038/sj.onc.1200898. PMID 9050999.
- Choi SS, Park SH, Kim UJ, Shin HS (1997). "Bfl-1, a Bcl-2-related gene, is the human homolog of the murine A1, and maps to chromosome 15q24.3". Mamm. Genome. 8 (10): 781–2. doi:10.1007/s003359900567. PMID 9321477.
- Hsu SY, Kaipia A, McGee E, et al. (1997). "Bok is a pro-apoptotic Bcl-2 protein with restricted expression in reproductive tissues and heterodimerizes with selective anti-apoptotic Bcl-2 family members". Proc. Natl. Acad. Sci. U.S.A. 94 (23): 12401–6. doi:10.1073/pnas.94.23.12401. PMC 24966. PMID 9356461.
- Takemoto Y, Furuta M, Sato M, et al. (1998). "Growth factor receptor-bound protein 2 (Grb2) association with hemopoietic specific protein 1: linkage between Lck and Grb2". J. Immunol. 161 (2): 625–30. PMID 9670936.
- Zong WX, Edelstein LC, Chen C, et al. (1999). "The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-kappaB that blocks TNFalpha-induced apoptosis". Genes Dev. 13 (4): 382–7. doi:10.1101/gad.13.4.382. PMC 316475. PMID 10049353.
- Holmgreen SP, Huang DC, Adams JM, Cory S (1999). "Survival activity of Bcl-2 homologs Bcl-w and A1 only partially correlates with their ability to bind pro-apoptotic family members". Cell Death Differ. 6 (6): 525–32. doi:10.1038/sj.cdd.4400519. PMID 10381646.
- Lee HH, Dadgostar H, Cheng Q, et al. (1999). "NF-kappaB-mediated up-regulation of Bcl-x and Bfl-1/A1 is required for CD40 survival signaling in B lymphocytes". Proc. Natl. Acad. Sci. U.S.A. 96 (16): 9136–41. doi:10.1073/pnas.96.16.9136. PMC 17745. PMID 10430908.
- Wang CY, Guttridge DC, Mayo MW, Baldwin AS (1999). "NF-kappaB induces expression of the Bcl-2 homologue A1/Bfl-1 to preferentially suppress chemotherapy-induced apoptosis". Mol. Cell. Biol. 19 (9): 5923–9. PMC 84448. PMID 10454539.
- Tomayko MM, Punt JA, Bolcavage JM, et al. (1999). "Expression of the Bcl-2 family member A1 is developmentally regulated in T cells". Int. Immunol. 11 (11): 1753–61. doi:10.1093/intimm/11.11.1753. PMID 10545479.
- Zhang H, Cowan-Jacob SW, Simonen M, et al. (2000). "Structural basis of BFL-1 for its interaction with BAX and its anti-apoptotic action in mammalian and yeast cells". J. Biol. Chem. 275 (15): 11092–9. doi:10.1074/jbc.275.15.11092. PMID 10753914.
- Duriez PJ, Wong F, Dorovini-Zis K, et al. (2000). "A1 functions at the mitochondria to delay endothelial apoptosis in response to tumor necrosis factor". J. Biol. Chem. 275 (24): 18099–107. doi:10.1074/jbc.M908925199. PMID 10849436.
- Harrington JJ, Sherf B, Rundlett S, et al. (2001). "Creation of genome-wide protein expression libraries using random activation of gene expression". Nat. Biotechnol. 19 (5): 440–5. doi:10.1038/88107. PMID 11329013.
- Bae J, Hsu SY, Leo CP, et al. (2001). "Underphosphorylated BAD interacts with diverse antiapoptotic Bcl-2 family proteins to regulate apoptosis". Apoptosis. 6 (5): 319–30. doi:10.1023/A:1011319901057. PMID 11483855.
- Akari H, Bour S, Kao S, et al. (2001). "The human immunodeficiency virus type 1 accessory protein Vpu induces apoptosis by suppressing the nuclear factor kappaB-dependent expression of antiapoptotic factors". J. Exp. Med. 194 (9): 1299–311. doi:10.1084/jem.194.9.1299. PMC 2195969. PMID 11696595.