BCDMH
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
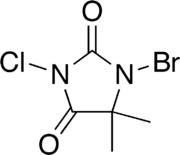
1-bromo-3-chloro-5,5-dimethylimidazolidine-2,4-dione | |
| Other names
bromochloro-5,5-dimethylhydantoin, BCDMH, agribrom, aquabrom, aquabrome, bromicide, di-halo, halogene T30, nylate, photobrome, slimicide 78P | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.036.557 |
PubChem CID |
|
| |
| |
| Properties | |
| C5H6BrClN2O2 | |
| Molar mass | 241.47 g/mol |
| Appearance | White solid |
| Density | 1.9 g/cm3 |
| Melting point | 159 to 163 °C (318 to 325 °F; 432 to 436 K) |
| 0.15 g/100 ml (25 °C) | |
| Hazards | |
| Main hazards | Flamability, Inhalation |
| Safety data sheet | External MSDS |
| R/S statement (outdated) | S8, S17, S26, S36, S37, S39, S41, S45 |
| NFPA 704 | |
| Flash point | Decomposes at 160°C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
1-Bromo-3-chloro-5,5-dimethylhydantoin (BCDMH) is a chemical structurally related to hydantoin. It is a white crystalline compound with a slight bromine and acetone odor and is insoluble in water, but soluble in acetone.
BCDMH is an excellent source of both chlorine and bromine as it reacts slowly with water releasing hypochlorous acid and hypobromous acid. It used as a chemical disinfectant used for recreational water and drinking water purification. BCDMH works in the following manner:[1]
The initial BCDMH reacts with water (R = Dimethylhydantoin):
Hypobromous acid partially dissociates in water:
- HOBr → H+ + OBr−
Hypobromous acid oxidizes the substrate, itself being reduced to bromide:
- HOBr + Live pathogens → Br− + Dead pathogens
The bromide ions are oxidized with the hypochlorous acid that was formed from the initial BCDMH:
- Br− + HOCl → HOBr + Cl−
This produces more hypobromous acid. However, the hypochlorous acid itself does act directly as a disinfectant in the process.
Preparation
This compound is prepared by first brominating, then chlorinating 5,5-dimethylhydantoin:[2]
References
- ↑ South Australian Health Commission, "Standard for the Operation of Swimming Pools and Spa Pools in South Australia", Supplement C: Bromine Disinfection, page 8. Retrieved on 2009-05-12.
- ↑ Yasukazu Ura, Gozyo Sakata, "Chloroamines", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a06_553

