Azoxystrobin
 | |
 | |
| Names | |
|---|---|
| IUPAC name
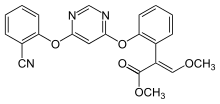
Methyl (2E)-2-(2-{[6-(2-cyanophenoxy)pyrimidin-4-yl]oxy}phenyl)-3-methoxyacrylate | |
| Other names
Azoxystrobine Heritage Amistar Quadris Bankit | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.127.964 |
| KEGG | |
PubChem CID |
|
| |
| |
| Properties | |
| C22H17N3O5 | |
| Molar mass | 403.388 |
| Density | 1.34 g/cm3 |
| Melting point | 116 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Azoxystrobin (brand name Amistar, Syngenta) is a systemic fungicide commonly used in agriculture. The substance is used as an active agent protecting plants and fruit/vegetables from fungal diseases.
Origin
Azoxystrobin was discovered during research on Oudemansiella mucida and Strobilurus tenacellus, which are small white or brown coloured mushrooms commonly found in European forests. Not bigger than a few centimeters, these mushrooms attracted attention of scientists because of their remarkable ability to defend themselves. Their defense mechanism is based on the secretion of two substances, strobilurin A and oudemansin A. These substances allow them to keep their competitors at a distance and kill them when in range. Observations of this mechanism led to research that resulted in the development of azoxystrobin. The molecule was synthetised for the first time by Dr. Christopher Godfrey at Jealott's Hill International Research Centre in Bracknell (UK).
Activity
After synthesizing experimental analogs of both substances (over 1400 were tested), azoxystrobin was found to be the most active and stable combination. The toxophore of azoxystrobin is the β-methoxyacrylate portion (shown in blue), which is present in the active compounds from both Oudemansiella mucida and Strobilurus tenacellus:[1]

These molecules bind very tightly to the Qo site of Complex III of the mitochondrial electron transport chain, thereby preventing production of ATP
Efficacy
Azoxystrobin possesses the broadest spectrum of activity of all known antifungals. It is the only counteragent that has the ability to protect against the four big groups of fungal and fungal-like diseases:
- Ascomycota: Septoria
- Deuteromycota: Pyricularia (rice harvesting)
- Basidiomycota: Stripe rust
- Oomycota: Water mould (grape harvesting)
Examples
Azoxystrobin is widely used in farming, particularly in wheat farming. Applying agents containing azoxystrobin provides protection against many types of diseases, including:
- Wheat septoria
- Septoria leaf spot
- Wheat leaf rust (Puccinia recondita)
- Rye leafrust (Puccinia triticina)
- Powdery mildew
- Downy mildew
- Stripe rust
- Haustorium
- Pyrenophora teres
Practical use
- Grain farming
- Banana transport
- grapes, both table & wine
- Purple wallboards (optiSHIELD AT, mixture of azoxystrobin and thiabendazole)
Ecotoxicology
Azoxystrobin has a favorable ecotoxicological profile, meeting the expectations of agricultural demand.
Azoxystrobin is broken down into the soil. Its toxicity is low for mammals, birds, bees, insects, and earthworms. "Azoxystrobin is classified as very toxic to aquatic organisms and [its main degradation product] R234886 as very harmful. [A recent] study shows that azoxystrobin and R234886 can leach through loamy soils for a long period of time following application of the pesticide and thereby pose a potential threat to vulnerable aquatic environments and drinking water resources." [2]
Food residues
In recent surveillance by the New Zealand Ministry for Primary Industries, 6 out of 24 olive oil samples contained residues of azoxystrobin or the fungicide propiconazole in excess of the maximum residue limit (MRL) set for agricultural chemicals. "When the processing factor is taken into account, these results indicate that the raw olives would have likely breached the MRLs for those compounds." In the Ministry's judgement, this violation did not pose health or food safety concerns.[3]
References
- ↑ Armstrong, Sarah; Clough, John (1 March 2009). "Crop protection chemicals". Education in Chemistry. Vol. 46 no. 2. Royal Society of Chemistry. pp. 52–56. Retrieved 19 June 2018.
- ↑ Jørgensen, Lisbeth Flindt; Jeanne Kjær; Preben Olsen; Annette Elisabeth Rosenbom (July 2012). "Leaching of azoxystrobin and its degradation product R234886 from Danish agricultural field sites". Chemosphere. 88 (5): 554–562. doi:10.1016/j.chemosphere.2012.03.027. PMID 22497784.
- ↑ Meister, Miriam (20 August 2012). "More good news on agricultural chemical good practice". Ministry for Primary Industries. Retrieved 23 November 2012.
External links
- National Pesticide Information Center
- Pesticide fact sheet
- Amistar (Product Description) - Syngenta Corporate
- Azoxystrobin in the Pesticide Properties DataBase (PPDB)