Alivecor
|
| |
| Private | |
| Industry | Medical Devices, Artificial Intelligence |
| Founded | 2011[1] |
| Founders | David Albert, Bruce Satchwell, Kim Barnett |
| Headquarters | Mountain View, California |
Key people | Vic Gundotra, CEO |
Number of employees | 50[1] |
| Website | alivecor.com |
AliveCor is a medical device and artificial intelligence company that sells ECG hardware and software for consumer mobile devices.[2][3] The company is the first to receive FDA-clearance for a medical-device accessory to the Apple Watch.[4][5]
History
The company was co-founded by David Albert, a medical doctor and former chief clinical scientist of cardiology at General Electric, along with scientists Bruce Satchwell and Kim Barnett.[6] Albert has been working on ECGs for handheld computers since 1990, when the first palm top computer was released by Hewlett-Packard.[7] He received a 1998 patent, along with Satchwell and Barnett, for wireless transmission of ECGs in handheld devices.[7]
In December 2010, Albert uploaded a YouTube video demonstrating a prototype of an iPhone ECG before attending the 2011 Consumer Electronics Show.[6] It received 100,000 views in two days and resulted in Albert appearing on Good Morning America, Fox & Friends and CNN. Shortly after, he was approached by venture capitalists and industry partners to fund the new company.[7] Alivecor received its first $3 million in financing in 2011.[6]
An FDA-approved smart-phone case that works as an ECG was released by Alivecor in 2012.[8] AliveCor ran two clinical trials to test the hardware and the app. The first study investigated how its single-lead ECG compared to a traditional 12-lead device.[9] The second study looked at whether 54 participants with no medical training could determine how to work the phone case.[10] The latter study was successful enough for FDA clearance and led to the diagnosis of two serious heart conditions.[10][8][11]
The company later released a credit-card sized device. The device required placing finger tips on its sensors for 30 seconds to get a medical-grade ECG reading. The data was sent to the cloud where it could be read by physicians.[12]
While its first ECG devices relied on doctors to analyze the readings, in 2015, the company received multiple clearances by the FDA to offer algorithmic analysis of readings to determine certain heart rhythm problems.[13]
The company hired former Google and Microsoft executive Vic Gundotra as its CEO in November 2016.[14] Gundotra subsequently hired two senior Google engineers to run hardware and software for Alivecor. Frank Petterson, an engineering lead at YouTube, became VP of engineering. Simon Prakash, a senior director at Google’s hardware unit, became VP of products and design.[15]
By 2017, the company's software was using neural networks to "train" its products to detect heart problems. The network uses four convolutional layers, with two tied to 300,000 parameters and takes about seven minutes to train on a new user. After about a month of product use, the software builds a heart profile of the specific user, a data-driven model that can detect problems, including how the electrical system within a heart is firing.[3]
AliveCor had recorded some 20 million ECGs by November 2017.[2]
By the end of 2017, the FDA had cleared the KardiaBand ECG reader as a medical device accessory to the Apple Watch. The KardiaBand had been approved for use in Europe earlier in the year.[4] A study by the Cleveland Clinic showed the KardiaBand could distinguish between atrial fibrillation and a normal heart rhythm with 93 percent sensitivity and 94 percent specificity; sensitivity increased to 99 percent with physician review of the reading.[16]
The company is conducting research with Columbia University[2] and the Mayo Clinic to determine if the KardiaBand can identify early warning signs of Long QT syndrome, a cause of sudden death, especially in young people.[17]
AliveCor presented research in March 2018 that it conducted with the Mayo Clinic showing that the KardiaBand can detect hyperkalemia, elevated levels of potassium in the blood, with 90% to 94% accuracy.[18] Existing tests for the condition require laboratory blood tests. To create the detection method, a dataset with 2 million ECGs linked to 4 million serum potassium values, collected over 23 years was analysed using artificial intelligence (AI). The company said it would pursue clinical trials and FDA-clearance for the detection method.[19]
As of 2018, Alivecor products had been tested in about 40 clinical studies published in peer-reviewed journals.[20]
Fast Company ranked Alivecor number one in artificial intelligence in its 2018 list of the "World's Most Innovative Companies."[21]
Products
KardiaBand
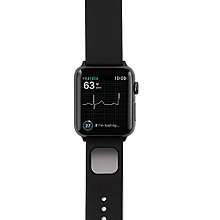
The KardiaBand is a wrist-band ECG reader for the Apple Watch, cleared by the FDA in 2017 as a medical device.[22][23] The device's SmartRhythm software uses artificial intelligence to continuously analyze data from the built-in heart-rate sensor and accelerometer to spot unexpected patterns.[2][24] The FDA has cleared the device's algorithm to detect atrial fibrillation or normal sinus rhythm.[24]
If an unusual pattern is detected by SmartRhythm, the user is alerted to take a 30-second ECG on the device by touching a wristband sensor for 30 seconds.[25] The device indicates to the user whether there is a normal or abnormal result. Full ECG readings can be shared externally, such as with a physician, for additional analysis.[26]
The product is connected to an iPhone app for storing data. It also works on its own, away from an iPhone or offline.[27]
KardiaMobile
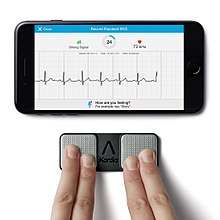
KardiaMobile is a smartphone-connected ECG. It is a FDA-cleared ECG medical device available for personal use.[28] The FDA has also cleared the device's algorithm to detect atrial fibrillation.[29] The user places their finger tips on a gum-stick sized device for about 30 seconds and the results are sent to their smart-phone (Android or iOS.)[12]
It was cleared by the FDA in 2012 for detecting atrial fibrillation and normal sinus rhythm. The results can be read by the user and also e-mailed to a physician.[28]
KardiaPro
KardiaPro is an artificial intelligence-enabled platform for doctors to monitor all their patients using Kardia devices, including for atrial fibrillation.[30] It provides a patient-by-patient dashboard of ECG data. The platform alerts doctors when a patient's device detects an abnormality so they can read the a full ECG.[3] The cloud-based platform can indicate signs of an oncoming stroke. KardiaPro also tracks patient risk factors, including weight, activity and blood pressure and analyzes them with AI to alert doctors to potential issues.[31]
Financing
Total investment by the end of 2017 was about $45 million.[17] The company raised $30 million in funding from medical-device maker Omron and the Mayo Clinic in 2017.[30] Previous investors include Khosla Ventures, Bold Capital Partners, and Burrill Life Sciences Capital.[32] Kearny Partners, Qualcomm and Oklahoma Life Science are also investors.[14]
References
- 1 2 Gibbs, Colin (3 August 2017). "Fierce 15: AliveCor". Fierce Wireless. Retrieved 30 March 2018.
- 1 2 3 4 "AliveCor: Most Innovative Company | Fast Company". Fast Company. Retrieved 2018-03-29.
- 1 2 3 "Ex-Googlers Build a Neural Network to Protect Your Heart". WIRED. Retrieved 2018-03-29.
- 1 2 "FDA clears AliveCor's Kardiaband as the first medical device accessory for the Apple Watch – TechCrunch". techcrunch.com. Retrieved 2018-03-29.
- ↑ "FDA clears first EKG band for the Apple Watch". Engadget. Retrieved 2018-03-30.
- 1 2 3 "iPhone ECG developer AliveCor raises $3 million". MobiHealthNews. 2011-08-02. Retrieved 2018-03-29.
- 1 2 3 "Ahead of His Time". MDDI Online. 2012-01-03. Retrieved 2018-03-29.
- 1 2 "This $200 iPhone Case Is An FDA-Approved EKG Machine". Co.Design. 2012-12-05. Retrieved 2018-03-29.
- ↑ Garabelli, Paul; Albert, David (11 May 2012). "Innovation Abstract # 12-IA-9662-HRS". Heart Rhythm Society's 33rd Annual Scientific Sessions.
- 1 2 Jayadewa, Chatu (2 April 2012). "AliveCor Takes Heart in Initial Findings of iPhone ECG Study". Scoop.it. Retrieved 13 April 2018.
- ↑ "Mobile Health Moves Forward: FDA Approves AliveCor's Heart Monitor For The iPhone – TechCrunch". techcrunch.com. Retrieved 2018-04-13.
- 1 2 "AliveCor raises $30 million for its credit card-sized heart monitor and app". VentureBeat. 2017-03-16. Retrieved 2018-03-29.
- ↑ "FDA clears AliveCor's automated detectors that record and display ECG rhythm". Medical Life Sciences News. 29 January 2015. Retrieved 13 April 2018.
- 1 2 "Mobile Heart Monitor Maker AliveCor Taps Former Google Exec Vic Gundotra as CEO". Recode. Retrieved 2018-03-30.
- ↑ "Vic Gundotra, Former Google+ Boss, Is Building a Heart Monitor for the Apple Watch". Recode. Retrieved 2018-03-30.
- ↑ "Study Suggests AliveCor KardiaBand for Apple Watch Can Be Used With AI Algorithm to Detect High Potassium". Retrieved 2018-03-30.
- 1 2 Farr, Christina (2017-07-19). "Former Google exec is teaming up with the Mayo Clinic to help prevent a major cause of sudden death". CNBC. Retrieved 2018-03-30.
- ↑ "Apple Watch may soon be able to detect hyperkalemia — no blood work required". 9to5Mac. 2018-03-12. Retrieved 2018-03-30.
- ↑ "Apple Watch wristband sensor claims to detect potassium in your blood — without needles". The Verge. Retrieved 2018-03-30.
- ↑ "AliveCor Research". alivecor.com. Retrieved 2018-04-02.
- ↑ "The 2018 Top 10 Most Innovative Companies by Sector: Artificial Intelligence | Fast Company". Fast Company. Retrieved 2018-04-02.
- ↑ "Your Apple Watch Could Tell You When You're Having a Stroke". Bloomberg.com. 2017-11-30. Retrieved 2018-03-30.
- ↑ Kincaid, Ellie. "FDA Clears AliveCor's Apple Watch ECG Device". Forbes. Retrieved 2018-03-30.
- 1 2 "This FDA-cleared Apple Watch band can actually tell you if your heart isn't working properly". Business Insider. Retrieved 2018-03-30.
- ↑ "AliveCor 'Kardia Band' Medical Grade EKG Analyzer for Apple Watch Receives FDA Approval". Retrieved 2018-03-29.
- ↑ "AliveCor launches ECG-sensing Apple Watch strap, SmartRhythm app". MobiHealthNews. 2017-11-30. Retrieved 2018-03-30.
- ↑ "Your Apple Watch can now be an FDA-cleared EKG device". CNET. 2017-11-30. Retrieved 2018-03-30.
- 1 2 "AliveCor | FierceWireless". www.fiercewireless.com. Retrieved 2018-03-29.
- ↑ "AliveCor gets FDA clearance for atrial fibrillation algorithm". MobiHealthNews. 2014-08-21. Retrieved 2018-04-02.
- 1 2 "Electrocardiogram app startup AliveCor raises $30M in new funding - SiliconANGLE". SiliconANGLE. 2017-03-16. Retrieved 2018-03-29.
- ↑ "AliveCor unveils an AI stroke prevention platform, inks $30 million from Omron and the Mayo Clinic – TechCrunch". techcrunch.com. Retrieved 2018-04-02.
- ↑ "AliveCor raises $30 million for its credit card-sized heart monitor and app". VentureBeat. 2017-03-16. Retrieved 2018-03-30.