Acid dye
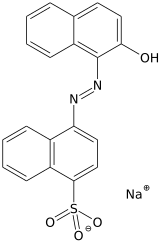
An acid dye is a dye that is typically applied to a textile at low pH. They are mainly used to dye wool, not cotton fabrics.[1] Some acid dyes are used as food colorants.[2]
Uses
Fibers
In the laboratory, home, or art studio, the acid used in the dye-bath is often vinegar (acetic acid) or citric acid. The uptake rate of the dye is controlled with the use of sodium chloride. In textiles, acid dyes are effective on protein fibers, i.e. animal hair fibers like wool, alpaca and mohair. They are also effective on silk.[3] They are effective in dyeing the synthetic fiber nylon, but of minimum interest in dyeing any other synthetic fibers.
Medical
In staining during microscopic examination for diagnosis or research, acid dyes are used to color basic tissue proteins. In contrast, basic dyes are used to stain cell nuclei and some other acidic components of tissues.[4]
Description
Acid dyes are generally divided into three classes which depend on fastness requirements, level dyeing properties and economy. The classes overlap and generally depend on type of fiber to be colored as well as the process used.[5]
Acid dyes affix to fibers by hydrogen bonding, Van der Waals forces[6] and ionic bonding. They are normally sold as the Sodium salt, therefore they are in solution anionic. Animal protein fibers and synthetic nylon fibers contain many cationic sites. Therefore, there is an attraction of anionic dye molecule to a cationic site on the fiber. The strength (fastness) of this bond is related to the tendency of the dye to remain dissolved in water over fixation to the fiber.
Structures
The chemistry of acid dyes is complex and diverse. Most acid dyes are related in basic structure to the following:
- Anthraquinone type: Many acid dyes are synthesized from chemical intermediates that form anthraquinone-like structures as their final state. Many blue dyes have this structure as their basic shape. The structure predominates in the leveling class of acid dye.
- Azo dyes: The structure of azo dyes contains the azo group (R-N=N−R. Most azo dyes are not acid dyes, but many acid dyes are azo dyes. Many acid dyes of the azo type are red in color.[7]
- Triarylmethane dye: These predominate in the milling class of dye. There are many yellow and green dyes commercially applied to fibers that are related to triphenylmethane.
Classes of acid dyes
Three typical classification of acid dyes are:[1]
- Leveling acid dyes: These dyes are of relatively low molecular weight, thus they migrate rapidly before fixation. Associated with their high mobility, leveling dyes exhibit low wet fastness therefore normally not suited for apparel fabric. These dyes are applied at low pH, often using sulfuric acid and sodium sulfate mixtures.
- Milling dyes: These dyes are of relatively high molecular weight, thus they migrate more slowly than leveling dyes. Associated with their low mobility, milling dyes exhibit better wet fastness than leveling dyes. These dyes are applied at higher pH, often using acetic acid.
- Metal complex acid dyes: More recent chemistry combined transition metals with dye precursors to produce metal complex acid dyes with the highest light fastness and wet fastness. These dyes are also very economical. They produce, however, duller shades.
References
- 1 2 Booth, Gerald (2000). Dyes, General Survey. Weinheim: Wiley-VCH. doi:10.1002/14356007.a09_073.
- ↑ Trowbridge Filippone, Peggy. "Food Color Additives". Retrieved September 8, 2016.
- ↑ "How Acid Dye Works". Retrieved September 8, 2016.
- ↑ Bruckner, Monica Z. "Basic Cellular Staining". Retrieved December 12, 2013.
- ↑ "Mechanism of Dyeing with Acid Dyes". Textile Learner. Mazharul Islam Kiron. Retrieved 2012-01-08.
- ↑ Clark, Jim (2012). "Intermolecular bonding - van der Waals forces". chemguide.co.uk. Retrieved 15 June 2014.
- ↑ Hunger, Klaus; Mischke, Peter; Rieper, Wolfgang; Raue, Roderich; Kunde, Klaus; Engel, Aloys (2005). "Azo Dyes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_245.
