APEX1
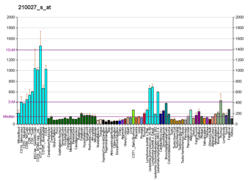
DNA-(apurinic or apyrimidinic site) lyase is an enzyme that in humans is encoded by the APEX1 gene.
Apurinic/apyrimidinic (AP) sites (also called "abasic sites") occur frequently in DNA molecules by spontaneous hydrolysis, by DNA damaging agents or by DNA glycosylases that remove specific abnormal bases. AP sites are pre-mutagenic lesions that can prevent normal DNA replication. All cells, from simple prokaryotes to humans, have evolved systems to identify and repair such sites. Class II AP endonucleases cleave the phosphodiester backbone 5' to the AP site, thereby initiating a process known as base excision repair (BER). The APEX gene (alternatively named APE1, HAP1, APEN) encodes the major AP endonuclease in human cells. Splice variants have been found for this gene; all encode the same protein.[5]
Interactions
APEX1 has been shown to interact with MUTYH,[6] Flap structure-specific endonuclease 1[7] and XRCC1.[8]
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000100823 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000035960 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ "Entrez Gene: APEX1 APEX nuclease (multifunctional DNA repair enzyme) 1".
- ↑ Parker, A; Gu Y; Mahoney W; Lee S H; Singh K K; Lu A L (February 2001). "Human homolog of the MutY repair protein (hMYH) physically interacts with proteins involved in long patch DNA base excision repair". J. Biol. Chem. United States. 276 (8): 5547–55. doi:10.1074/jbc.M008463200. ISSN 0021-9258. PMID 11092888.
- ↑ Dianova, I I; Bohr V A; Dianov G L (October 2001). "Interaction of human AP endonuclease 1 with flap endonuclease 1 and proliferating cell nuclear antigen involved in long-patch base excision repair". Biochemistry. United States. 40 (42): 12639–44. doi:10.1021/bi011117i. ISSN 0006-2960. PMID 11601988.
- ↑ Vidal, A E; Boiteux S; Hickson I D; Radicella J P (November 2001). "XRCC1 coordinates the initial and late stages of DNA abasic site repair through protein-protein interactions". EMBO J. England. 20 (22): 6530–9. doi:10.1093/emboj/20.22.6530. ISSN 0261-4189. PMC 125722. PMID 11707423.
Further reading
- Mol CD, Hosfield DJ, Tainer JA (2000). "Abasic site recognition by two apurinic/apyrimidinic endonuclease families in DNA base excision repair: the 3' ends justify the means". Mutat. Res. 460 (3–4): 211–29. doi:10.1016/s0921-8777(00)00028-8. PMID 10946230.
- Fritz G (2000). "Human APE/Ref-1 protein". Int. J. Biochem. Cell Biol. 32 (9): 925–9. doi:10.1016/S1357-2725(00)00045-5. PMID 11084372.
- Fritz G, Grösch S, Tomicic M, Kaina B (2003). "APE/Ref-1 and the mammalian response to genotoxic stress". Toxicology. 193 (1–2): 67–78. doi:10.1016/S0300-483X(03)00290-7. PMID 14599768.
- Tell G, Damante G, Caldwell D, Kelley MR (2005). "The intracellular localization of APE1/Ref-1: more than a passive phenomenon?". Antioxid. Redox Signal. 7 (3–4): 367–84. doi:10.1089/ars.2005.7.367. PMID 15706084.
- Hung RJ, Hall J, Brennan P, Boffetta P (2006). "Genetic polymorphisms in the base excision repair pathway and cancer risk: a HuGE review". Am. J. Epidemiol. 162 (10): 925–42. doi:10.1093/aje/kwi318. PMID 16221808.
- Dyrkheeva NS, Khodyreva SN, Lavrik OI (2007). "[Multifunctional human apurinic/apyrimidinic endonuclease 1: the role of additional functions]". Mol. Biol. (Mosk.). 41 (3): 450–66. PMID 17685223.
- Harrison L, Ascione G, Menninger JC, et al. (1993). "Human apurinic endonuclease gene (APE): structure and genomic mapping (chromosome 14q11.2-12)". Hum. Mol. Genet. 1 (9): 677–80. doi:10.1093/hmg/1.9.677. PMID 1284593.
- Cheng XB, Bunville J, Patterson TA (1992). "Nucleotide sequence of a cDNA for an apurinic/apyrimidinic endonuclease from HeLa cells". Nucleic Acids Res. 20 (2): 370. doi:10.1093/nar/20.2.370. PMC 310384. PMID 1371347.
- Xanthoudakis S, Miao G, Wang F, et al. (1992). "Redox activation of Fos-Jun DNA binding activity is mediated by a DNA repair enzyme". EMBO J. 11 (9): 3323–35. PMC 556867. PMID 1380454.
- Zhao B, Grandy DK, Hagerup JM, et al. (1992). "The human gene for apurinic/apyrimidinic endonuclease (HAP1): sequence and localization to chromosome 14 band q12". Nucleic Acids Res. 20 (15): 4097–8. doi:10.1093/nar/20.15.4097. PMC 334100. PMID 1380694.
- Robson CN, Hochhauser D, Craig R, et al. (1992). "Structure of the human DNA repair gene HAP1 and its localisation to chromosome 14q 11.2-12". Nucleic Acids Res. 20 (17): 4417–21. doi:10.1093/nar/20.17.4417. PMC 334166. PMID 1383925.
- Seki S, Hatsushika M, Watanabe S, et al. (1992). "cDNA cloning, sequencing, expression and possible domain structure of human APEX nuclease homologous to Escherichia coli exonuclease III". Biochim. Biophys. Acta. 1131 (3): 287–99. doi:10.1016/0167-4781(92)90027-w. PMID 1627644.
- Robson CN, Hickson ID (1991). "Isolation of cDNA clones encoding a human apurinic/apyrimidinic endonuclease that corrects DNA repair and mutagenesis defects in E. coli xth (exonuclease III) mutants". Nucleic Acids Res. 19 (20): 5519–23. doi:10.1093/nar/19.20.5519. PMC 328951. PMID 1719477.
- Demple B, Herman T, Chen DS (1992). "Cloning and expression of APE, the cDNA encoding the major human apurinic endonuclease: definition of a family of DNA repair enzymes". Proc. Natl. Acad. Sci. U.S.A. 88 (24): 11450–4. Bibcode:1991PNAS...8811450D. doi:10.1073/pnas.88.24.11450. PMC 53153. PMID 1722334.
- Okazaki T, Chung U, Nishishita T, et al. (1994). "A redox factor protein, ref1, is involved in negative gene regulation by extracellular calcium". J. Biol. Chem. 269 (45): 27855–62. PMID 7961715.
- Akiyama K, Seki S, Oshida T, Yoshida MC (1994). "Structure, promoter analysis and chromosomal assignment of the human APEX gene". Biochim. Biophys. Acta. 1219 (1): 15–25. doi:10.1016/0167-4781(94)90241-0. PMID 8086453.
- Andersson B, Wentland MA, Ricafrente JY, et al. (1996). "A "double adaptor" method for improved shotgun library construction". Anal. Biochem. 236 (1): 107–13. doi:10.1006/abio.1996.0138. PMID 8619474.
- Chung U, Igarashi T, Nishishita T, et al. (1996). "The interaction between Ku antigen and REF1 protein mediates negative gene regulation by extracellular calcium". J. Biol. Chem. 271 (15): 8593–8. doi:10.1074/jbc.271.15.8593. PMID 8621488.
- Rothwell DG, Hickson ID (1996). "Asparagine 212 is essential for abasic site recognition by the human DNA repair endonuclease HAP1". Nucleic Acids Res. 24 (21): 4217–21. doi:10.1093/nar/24.21.4217. PMC 146231. PMID 8932375.
- Izumi T, Henner WD, Mitra S (1997). "Negative regulation of the major human AP-endonuclease, a multifunctional protein". Biochemistry. 35 (47): 14679–83. doi:10.1021/bi961995u. PMID 8942627.
External links
- Human APEX1 genome location and APEX1 gene details page in the UCSC Genome Browser.