Bisphenol A
 | |
 | |
| Names | |
|---|---|
| IUPAC name
4,4'-(propane-2,2-diyl)diphenol | |
| Other names
BPA, p,p'-Isopropylidenebisphenol, 2,2-Bis(4-hydroxyphenyl)propane | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.001.133 |
| EC Number | 201-245-8 |
| KEGG | |
PubChem CID |
|
| RTECS number | SL6300000 |
| UNII | |
| UN number | 2430 |
| |
| |
| Properties | |
| C15H16O2 | |
| Molar mass | 228.29 g·mol−1 |
| Appearance | White solid |
| Density | 1.20 g/cm³ |
| Melting point | 158 to 159 °C (316 to 318 °F; 431 to 432 K) |
| Boiling point | 220 °C (428 °F; 493 K) 4 mmHg |
| 120–300 ppm (21.5 °C) | |
| Vapor pressure | 5×10−6 Pa (25 °C)[1] |
| Hazards | |
| R-phrases (outdated) | R36 R37 R38 R43 |
| S-phrases (outdated) | S24 S26 S37 |
| NFPA 704 | |
| Flash point | 227 °C (441 °F; 500 K) |
| 600 °C (1,112 °F; 873 K) | |
| Related compounds | |
Related phenols |
Bisphenol S |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
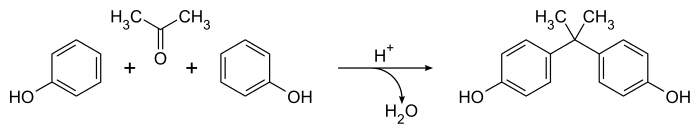
Bisphenol A (BPA) is an organic synthetic compound with the chemical formula (CH3)2C(C6H4OH)2 belonging to the group of diphenylmethane derivatives and bisphenols, with two hydroxyphenyl groups. It is a colorless solid that is soluble in organic solvents, but poorly soluble in water. It has been in commercial use since 1957.
BPA is a starting material for the synthesis of plastics, primarily certain polycarbonates and epoxy resins, as well as some polysulfones and certain niche materials. BPA-based plastic is clear and tough, and is made into a variety of common consumer goods, such as plastic bottles including water bottles, sports equipment, CDs, and DVDs. Epoxy resins containing BPA are used to line water pipes, as coatings on the inside of many food and beverage cans and in making thermal paper such as that used in sales receipts.[2] In 2015, an estimated 4 million tonnes of BPA chemical were produced for manufacturing polycarbonate plastic, making it one of the highest volume of chemicals produced worldwide.[3]
BPA is a xenoestrogen, exhibiting estrogen-mimicking, hormone-like properties[4] that raise concern about its suitability in some consumer products and food containers. Since 2008, several governments have investigated its safety, which prompted some retailers to withdraw polycarbonate products. The U.S. Food and Drug Administration (FDA) has ended its authorization of the use of BPA in baby bottles and infant formula packaging, based on market abandonment, not safety.[5] The European Union and Canada have banned BPA use in baby bottles.
Production
World production capacity of Bisphenol A was 1 million tons in the 1980s,[6] and more than 2.2 million tons in 2009.[7] It is a high production volume chemical. In 2003, U.S. consumption was 856,000 tons, 72% of which used to make polycarbonate plastic and 21% going into epoxy resins.[8] In the U.S., less than 5% of the BPA produced is used in food contact applications,[9] but remains in the canned food industry and printing applications such as sales receipts.[10][11]
Bisphenol A was first synthesized by the Russian chemist Alexander Dianin in 1891.[12][13] This compound is synthesized by the condensation of acetone (hence the suffix A in the name)[14] with two equivalents of phenol. The reaction is catalyzed by a strong acid, such as hydrochloric acid (HCl) or a sulfonated polystyrene resin. Industrially, a large excess of phenol is used to ensure full condensation; the product mixture of the cumene process (acetone and phenol) may also be used as starting material:[6]
A large number of ketones undergo analogous condensation reactions. Commercial production of BPA requires distillation – either extraction of BPA from many resinous byproducts under high vacuum or solvent-based extraction using additional phenol followed by distillation.[6]
Uses
BPA is a precursor to other chemicals, predominantly polymers. Otherwise, BPA is uncommon in industry and in the laboratory.
Polycarbonates
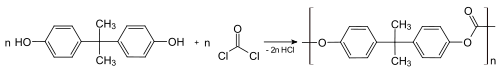
Bisphenol A is used primarily to make plastics. Bisphenol A based plastics have been in commercial use since 1957.[15] It is a key ingredient in polymers that are used to make adhesives, containers, electronic coatings, boat hulls. In terms of consumer goods, reusable drink containers, food storage containers, canned foods, children's toys, and even receipts from stores. At least 3.6 million tonnes (8 billion pounds) of BPA are used by manufacturers yearly.[16] However, it is estimated that the global annual output of BPA 6.8 million tonnes.[17] It is a key monomer in production of epoxy resins[18][19] Bisphenol A and phosgene react to give polycarbonate under biphasic conditions; the hydrochloric acid is scavenged with aqueous base:
In principle diphenyl carbonate may be used in place of phosgene. Phenol is eliminated instead of hydrochloric acid. This transesterification process avoids the toxicity and handling of phosgene.[20]
Epoxy and vinyl ester resins
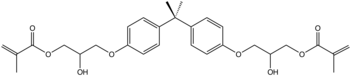
Vinyl ester resins and some epoxy resins are produced using BPA
Other uses of BPA
BPA is also used in the synthesis of polysulfones and some Polyether ether ketones. It is an antioxidant in some plasticizers, and as a polymerization inhibitor in PVC.
Identification in plastics
Plastic packaging is split into seven broad classes for recycling purposes by a Plastic identification code. As of 2014 there are no BPA labeling requirements for plastics in the U.S. "In general, plastics that are marked with Resin Identification Codes 1, 2, 4, 5, and 6 are very unlikely to contain BPA. Some, but not all, plastics that are marked with the Resin Identification Code 7 may be made with BPA."[22] Type 7 is the catch-all "other" class, and some type 7 plastics, such as polycarbonate (sometimes identified with the letters "PC" near the recycling symbol) and epoxy resins, are made from bisphenol A monomer.[6][23] Type 3 (PVC) may contain bisphenol A as an antioxidant in "flexible PVC" softened by plasticizers,[6] but not rigid PVC such as pipe, windows, and siding.
History
Bisphenol A was discovered in 1891 by Russian chemist Aleksandr Dianin.[24]
Based on research by chemists at Bayer and General Electric, BPA has been used since the 1950s to harden polycarbonate plastics, and make epoxy resin, which is contained in the lining of food and beverage containers.[25][26]
In the early 1930s, the British biochemist Edward Charles Dodds tested BPA as an artificial estrogen, but found it to be 37,000 times less effective than estradiol.[27][28][29] Dodds eventually developed a structurally similar[30] compound, diethylstilbestrol (DES), which was used as a synthetic estrogen drug in women and animals until it was banned due to its risk of causing cancer; the ban on use of DES in humans came in 1971 and in animals, in 1979.[27] BPA was never used as a drug.[27] BPA's ability to mimic the effects of natural estrogen derive from the similarity of phenol groups on both BPA and estradiol, which enable this synthetic molecule to trigger estrogenic pathways in the body.[31] Typically phenol-containing molecules similar to BPA are known to exert weak estrogenic activities, thus it is also considered an endocrine disrupter (ED) and estrogenic chemical.[32] Xenoestrogens is another category the chemical BPA fits under because of its capability to interrupt the network that regulates the signals which control the reproductive development in humans and animals.[33]
BPA has been found to bind to both of the nuclear estrogen receptors (ERs), ERα and ERβ.[30] It is 1000- to 2000-fold less potent than estradiol.[30] BPA can both mimic the action of estrogen and antagonize estrogen, indicating that it is a selective estrogen receptor modulator (SERM) or partial agonist of the ER.[30] At high concentrations, BPA also binds to and acts as an antagonist of the androgen receptor (AR).[30] In addition to receptor binding, the compound has been found to affect Leydig cell steroidogenesis, including affecting 17α-hydroxylase/17,20 lyase and aromatase expression and interfering with LH receptor-ligand binding.[30]
In 1997, adverse effects of low-dose BPA exposure in laboratory animals were first proposed.[34] Modern studies began finding possible connections to health issues caused by exposure to BPA during pregnancy and during development. See US public health regulatory history and Chemical manufacturers reactions to bans. As of 2014, research and debates are ongoing as to whether BPA should be banned or not.
A 2007 study investigated the interaction between bisphenol A's and estrogen-related receptor γ (ERR-γ). This orphan receptor (endogenous ligand unknown) behaves as a constitutive activator of transcription. BPA seems to bind strongly to ERR-γ (dissociation constant = 5.5 nM), but only weakly to the ER.[35] BPA binding to ERR-γ preserves its basal constitutive activity.[35] It can also protect it from deactivation from the SERM 4-hydroxytamoxifen (afimoxifene).[35] This may be the mechanism by which BPA acts as a xenoestrogen.[35] Different expression of ERR-γ in different parts of the body may account for variations in bisphenol A effects. BPA has also been found to act as an agonist of the GPER (GPR30).[36]
Health effects

According to the European Food Safety Authority "BPA poses no health risk to consumers of any age group (including unborn children, infants and adolescents) at current exposure levels".[37] But in 2017 the European Chemicals Agency concluded that BPA should be listed as a substance of very high concern due to its properties as an endocrine disruptor.[38]
In 2012, the United States' Food and Drug Administration (FDA) banned the use of BPA in baby bottles.[39]
The Environmental Protection Agency (EPA) also holds the position that BPA is not a health concern. In 2011, Andrew Wadge, the chief scientist of the United Kingdom's Food Standards Agency, commented on a 2011 U.S. study on dietary exposure of adult humans to BPA,[40] saying, "This corroborates other independent studies and adds to the evidence that BPA is rapidly absorbed, detoxified, and eliminated from humans – therefore is not a health concern."[41]
The Endocrine Society said in 2015 that the results of ongoing laboratory research gave grounds for concern about the potential hazards of endocrine-disrupting chemicals – including BPA – in the environment, and that on the basis of the precautionary principle these substances should continue to be assessed and tightly regulated.[42] A 2016 review of the literature said that the potential harms caused by BPA were a topic of scientific debate and that further investigation was a priority because of the association between BPA exposure and adverse human health effects including reproductive and developmental effects and metabolic disease.[43]
Environmental effects
In 2010, the U.S. Environmental Protection Agency reported that over one million pounds of BPA are released into the environment annually.[44] BPA can be released into the environment by both pre-consumer and post-consumer leaching. Common routes of introduction from the pre-consumer perspective into the environment are directly from chemical plastics, coat and staining manufacturers, foundries who use BPA in casting sand, or transport of BPA and BPA-containing products .[45][46] Post-consumer BPA waste comes from effluent discharge from municipal wastewater treatment plants, irrigation pipes used in agriculture, ocean-borne plastic trash, indirect leaching from plastic, paper, and metal waste in landfills, and paper or material recycling companies.[45][46][47] Despite a rapid soil and water half-life of 4.5 days, and an air half-life of less than one day, BPA's ubiquity makes it an important pollutant. BPA has a low rate of evaporation from water and soil, which presents issues, despite its biodegradability and low concern for bio-accumulation. BPA has low volatility in the atmosphere and a low vapor pressure between 5.00 and 5.32 Pascals. BPA has a high water solubility of about 120 mg/L and most of its reactions in the environment are aqueous. An interesting fact is that BPA dust is flammable if ignited, but it has a minimal explosive concentration in air.[48] Also, in aqueous solutions, BPA absorbs at wavelengths greater than 250 nm.[49]
The ubiquitous nature of BPA makes the compound an important pollutant to study as it has been shown to interfere with nitrogen fixation at the roots of leguminous plants associated with the bacterial symbiont Sinorhizobium meliloti.[50] A 2013 study also observed changes in plant health due to BPA exposure. The study exposed soybean seedlings to various concentrations of BPA and saw changes in root growth, nitrate production, ammonium production, and changes in the activities of nitrate reductase and nitrite reductase. At low doses of BPA, the growth of roots were improved, the amount of nitrate in roots increased, the amount of ammonium in roots decreased, and the nitrate and nitrite reductase activities remained unchanged. However, at considerably higher concentrations of BPA, the opposite effects were seen for all but an increase in nitrate concentration and a decrease in nitrite and nitrate reductase activities.[51] Nitrogen is both a plant nutritional substance, but also the basis of growth and development in plants. Changing concentrations of BPA can be harmful to the ecology of an ecosystem, as well as to humans if the plants are produced to be consumed.
The amount of absorbed BPA on sediment was also seen to decrease with increases in temperature, as demonstrated by a study in 2006 with various plants from the Xiangjiang River in Central-South China. In general, as temperature increases, the water solubility of a compound increases. Therefore, the amount of sorbate that enters the solid phase will lower at the equilibrium point. It was also observed that the adsorption process of BPA on sediment is exothermic, the molar formation enthalpy, ΔH°, was negative, the free energy ΔG°, was negative, and the molar entropy, ΔS°, was positive. This indicates that the adsorption of BPA is driven by enthalpy. The adsorption of BPA has also been observed to decrease with increasing pH.[52]
A 2005 study conducted in the United States had found that 91–98% of BPA may be removed from water during treatment at municipal water treatment plants.[53] A more detailed explanation of aqueous reactions of BPA can be observed in the Degradation of BPA section below. Nevertheless, a 2009 meta-analysis of BPA in the surface water system showed BPA present in surface water and sediment in the U.S. and Europe.[54] According to Environment Canada in 2011, "BPA can currently be found in municipal wastewater. […]initial assessment shows that at low levels, bisphenol A can harm fish and organisms over time."[55]
BPA affects growth, reproduction, and development in aquatic organisms. Among freshwater organisms, fish appear to be the most sensitive species. Evidence of endocrine-related effects in fish, aquatic invertebrates, amphibians, and reptiles has been reported at environmentally relevant exposure levels lower than those required for acute toxicity. There is a widespread variation in reported values for endocrine-related effects, but many fall in the range of 1μg/L to 1 mg/L.[9]
A 2009 review of the biological impacts of plasticizers on wildlife published by the Royal Society with a focus on aquatic and terrestrial annelids, molluscs, crustaceans, insects, fish and amphibians concluded that BPA affects reproduction in all studied animal groups, impairs development in crustaceans and amphibians and induces genetic aberrations.[56]
References
- ↑ "Chemical Fact Sheet – Cas #80057 CASRN 80-05-7". speclab.com. 1 April 2012.
- ↑ Pivnenko, K.; Pedersen, G. A.; Eriksson, E.; Astrup, T. F. (2015-10-01). "Bisphenol A and its structural analogues in household waste paper" (Submitted manuscript). Waste Management. 44: 39–47. doi:10.1016/j.wasman.2015.07.017. PMID 26194879.
- ↑ http://www.conseil-constitutionnel.fr/conseil-constitutionnel/francais/videos/2015/septembre/affaire-n-2015-480-qpc.144326.html
- ↑ Egan, Michael (March 2014). "Sarah A. Vogel. Is It Safe? BPA and the Struggle to Define the Safety of Chemicals. xxi + 304 pp., illus., index. Berkeley: University of California Press, 2013". Isis. 105 (1): 254. doi:10.1086/676809. ISSN 0021-1753.
- ↑ "Bisphenol A (BPA): Use in Food Contact Application". Fda.gov. November 2014. Retrieved June 21, 2018.
- 1 2 3 4 5 Fiege H; Voges H-W; Hamamoto T; Umemura S; Iwata T; Miki H; Fujita Y; Buysch H-J; Garbe D; Paulus W (2000). "Phenol Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_313. ISBN 978-3527306732.
- ↑ "Experts demand European action on plastics chemical". Reuters. 22 June 2010.
- ↑ National Toxicology Program, U.S. Department of Health and Human Services (September 2008). "CERHR Expert Panel Report for Bisphenol A" (PDF). Retrieved 2013-05-31.
- 1 2 "Bisphenol A Action Plan" (PDF). U.S. Environmental Protection Agency. 29 March 2010. Retrieved 12 April 2010.
- ↑ "Concern over canned foods". Consumer Reports. December 2009. Retrieved 2 February 2012.
- ↑ "Soaring BPA Levels Found in People Who Eat Canned Foods". Fox News Channel. 23 November 2011.
- ↑ А. П. Дианин (A. P. Dianin) (1891). "О продуктах конденсации кетонов с фенолами" [On condensation products of ketones with phenols]. Журнал Русского Физико-Химического Общества (Journal of the Russian Physical-Chemical Society). 23: 488–517, 523–546, 601–611. See especially p. 492.
- ↑ Zincke, T. (1905). "Ueber die Einwirkung von Brom und von Chlor auf Phenole: Substitutionsprodukte, Pseudobromide und Pseudochloride". Justus Liebigs Annalen der Chemie. 343: 75–99. doi:10.1002/jlac.19053430106.
- ↑ Uglea, Constantin V.; Negulescu, Ioan I. (1991). Synthesis and Characterization of Oligomers. CRC Press. p. 103. ISBN 978-0-8493-4954-6.
- ↑ "Bisphenol A Information Sheet" (PDF). Bisphenol A Global Industry Group. October 2002. Retrieved 7 December 2010.
- ↑ "Studies Report More Harmful Effects From BPA". U.S. News & World Report. 10 June 2009. Retrieved 28 October 2010.
- ↑ Zhang, Jiazhi; Li, Xingyi; Zhou, Li; Wang, Lihong; Zhou, Qing; Huang, Xiaohua (2016-03-31). "Analysis of effects of a new environmental pollutant, bisphenol A, on antioxidant systems in soybean roots at different growth stages". Scientific Reports. 6: 23782. Bibcode:2016NatSR...623782Z. doi:10.1038/srep23782. ISSN 2045-2322. PMC 4815016. PMID 27030053.
- ↑ Kroschwitz, Jacqueline I. Kirk-Othmer encyclopedia of chemical technology. 5 (5 ed.). p. 8. ISBN 978-0-471-52695-7.
- ↑ "Polycarbonate (PC) Polymer Resin". Alliance Polymers, Inc. Archived from the original on 21 September 2009. Retrieved 2 August 2009.
- ↑ Wittcoff, Harold; Reuben, B. G.; Plotkin, Jeffrey S. (2004). Industrial Organic Chemicals. Wiley-IEEE. p. 278. ISBN 978-0-471-44385-8. Retrieved 1 February 2012.
- ↑ Pham, Ha Q.; Marks, Maurice J. (2012). Epoxy Resins. Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a09_547.pub2. ISBN 978-3527306732.
- ↑ "Bisphenol A (BPA) Information for Parents". Hhs.gov. 15 January 2010. Retrieved 23 October 2011.
- ↑ Biello D (19 February 2008). "Plastic (not) fantastic: Food containers leach a potentially harmful chemical". Scientific American. 2. Retrieved 9 April 2008.
- ↑ See:
- А. Дианина (1891) "О продуктахъ конденсацiи кетоновъ съ фенолами" (On condensation products of ketones with phenols), Журнал Русского физико-химического общества (Journal of the Russian Physical Chemistry Society), 23 : 488-517, 523–546, 601–611 ; see especially pages 491-493 ("Диметилдифеиолметаиь" (dimethyldiphenolmethane)).
- Reprinted in condensed form in: A. Dianin (1892) "Condensationsproducte aus Ketonen und Phenolen" (Condensation products of ketones and phenols), Berichte der Deutschen chemischen Gesellschaft zu Berlin, 25, part 3 : 334-337.
- ↑ Heather Caliendo for PlasticsToday – Packaging Digest, 20 June 2012 History of BPA Archived 12 June 2013 at the Wayback Machine.
- ↑ Walsh B (1 April 2010). "The Perils of Plastic – Environmental Toxins – TIME". Time. Retrieved 2 July 2010.
- 1 2 3 Vogel SA (2009). "The Politics of Plastics: The Making and Unmaking of Bisphenol A "Safety". Am J Public Health. 99 (S3): S559–S566. doi:10.2105/AJPH.2008.159228. PMC 2774166. PMID 19890158.
- ↑ Dodds EC, Lawson W (1936). "Synthetic Œstrogenic Agents without the Phenanthrene Nucleus". Nature. 137 (3476): 996. Bibcode:1936Natur.137..996D. doi:10.1038/137996a0.
- ↑ Dodds E. C.; Lawson W. (1938). "Molecular Structure in Relation to Oestrogenic Activity. Compounds without a Phenanthrene Nucleus". Proceedings of the Royal Society of London B: Biological Sciences. 125 (839): 222–232. Bibcode:1938RSPSB.125..222D. doi:10.1098/rspb.1938.0023.
- 1 2 3 4 5 6 Hejmej, Anna; Kotula-Balak, Magorzata; Bilinsk, Barbara (2011). Antiandrogenic and Estrogenic Compounds: Effect on Development and Function of Male Reproductive System. Steroids – Clinical Aspect. doi:10.5772/28538. ISBN 978-953-307-705-5.
- ↑ Kwon J.H.; Katz L.E.; Liljestrand H.M. (2007). "Modeling binding equilibrium in a competitive estrogen receptor binding assay". Chemosphere. 69 (7): 1025–1031. Bibcode:2007Chmsp..69.1025K. doi:10.1016/j.chemosphere.2007.04.047. PMID 17559906.
- ↑ Ahmed, R. A. M. (2014). "Effect of prenatal exposure to Bisphenol A on the vagina of albino rats: immunohistochemical and ultrastructural study". Folia Morphologica. 73 (4): 399–408. doi:10.5603/FM.2014.0061. PMID 25448896.
- ↑ Ramos, J.G. (2003). "Bisphenol A induces both transient and permanent histofunctional alterations of the hypothalamic-pituitary-gonadal axis in prenatally exposed male rats". Endocrinology. 144 (7): 3206–3215. doi:10.1210/en.2002-0198. PMID 12810577.
- ↑ Erickson BE (2 June 2008). "Bisphenol A under scrutiny". Chemical and Engineering News. 86 (22): 36–39. doi:10.1021/cen-v086n022.p036.
- 1 2 3 4 Matsushima A, Kakuta Y, Teramoto T, Koshiba T, Liu X, Okada H, Tokunaga T, Kawabata S, Kimura M, Shimohigashi Y (October 2007). "Structural evidence for endocrine disruptor bisphenol A binding to human nuclear receptor ERR gamma". J. Biochem. 142 (4): 517–24. doi:10.1093/jb/mvm158. PMID 17761695.
- ↑ Prossnitz, Eric R.; Barton, Matthias (2014). "Estrogen biology: New insights into GPER function and clinical opportunities". Molecular and Cellular Endocrinology. 389 (1–2): 71–83. doi:10.1016/j.mce.2014.02.002. ISSN 0303-7207. PMC 4040308. PMID 24530924.
- ↑ "Bisphenol A". European Food Safety Authority. 2015. Lay summary.
- ↑ "MSC unanimously agrees that Bisphenol A is an endocrine disruptor - All news - ECHA". echa.europa.eu. Retrieved 2017-06-19.
- ↑ Mirmira, P; Evans-Molina, C (2014). "Bisphenol A, obesity, and type 2 diabetes mellitus: genuine concern or unnecessary preoccupation?". Translational Research: The Journal of Laboratory and Clinical Medicine (Review). 164 (1): 13–21. doi:10.1016/j.trsl.2014.03.003. hdl:1805/8373. PMC 4058392. PMID 24686036.
- ↑ Teeguarden JG, Calafat AM, Ye X, Doerge DR, Churchwell MI, Gunawan R, Graham MK (September 2011). "Twenty-four hour human urine and serum profiles of bisphenol A during high-dietary exposure". Toxicological Sciences. 123 (1): 48–57. doi:10.1093/toxsci/kfr160. PMID 21705716.
- ↑ Wage, Andrew (27 July 2011). "Small pond, same big issues". FSA. Archived from the original on 10 September 2011. Retrieved 3 August 2011.
- ↑ Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT (2015). "Executive Summary to EDC-2: The Endocrine Society's Second Scientific Statement on Endocrine-Disrupting Chemicals". Endocr. Rev. 36 (6): 593–602. doi:10.1210/er.2015-1093. PMC 4702495. PMID 26414233.
- ↑ Giulivo M, Lopez de Alda M, Capri E, Barceló D (2016). "Human exposure to endocrine disrupting compounds: Their role in reproductive systems, metabolic syndrome and breast cancer. A review". Environ. Res. (Review). 151: 251–264. Bibcode:2016ER....151..251G. doi:10.1016/j.envres.2016.07.011. PMID 27504873.
- ↑ Erler C, Novak J (October 2010). "Bisphenol a exposure: human risk and health policy". J Pediatr Nurs. 25 (5): 400–7. doi:10.1016/j.pedn.2009.05.006. PMID 20816563.
- 1 2 Corrales, Jone; Kristofco, Lauren A.; Steele, W. Baylor; Yates, Brian S.; Breed, Christopher S.; Williams, E. Spencer; Brooks, Bryan W. (2015-07-29). "Global Assessment of Bisphenol A in the Environment". Dose-Response. 13 (3): 1559325815598308. doi:10.1177/1559325815598308. ISSN 1559-3258. PMC 4674187. PMID 26674671.
- 1 2 EPA (26 July 2011). "Testing of Bisphenol A, Advance notice of proposed rulemaking (ANPRM)". Federal Register /Vol. 76, No. 143 / Proposed Rules. Federal Register. Retrieved 8 May 2017.
- ↑ "Plastic Breaks Down in Ocean, After All -- And Fast". news.nationalgeographic.com. Retrieved 2017-11-27.
- ↑ Rost, John M. (September 2012). "Risk-Based Green Screen Assessment of Bisphenol A" (PDF). Gradient Corp.
- ↑ Abo, Rudy (September 2016). "Optimized photodegradation of Bisphenol A in water using ZnO, TiO2, and SnO2 and photocatalysts under UV radiation as a decontamination procedure" (PDF). Drinking Water Engineering and Science. 9 (2): 27–35.
- ↑ Fox, Jennifer E.; Gulledge, Jay; Engelhaupt, Erika; Burow, Matthew E.; McLachlan, John A. (2007-06-12). "Pesticides reduce symbiotic efficiency of nitrogen-fixing rhizobia and host plants". Proceedings of the National Academy of Sciences. 104 (24): 10282–10287. Bibcode:2007PNAS..10410282F. doi:10.1073/pnas.0611710104. ISSN 0027-8424. PMC 1885820. PMID 17548832.
- ↑ Sun, Hai; Wang, Lihong; Zhou, Qing (January 2013). "Effects of bisphenol A on growth and nitrogen nutrition of roots of soybean seedlings". Environmental Toxicology and Chemistry. 32 (1): 174–180. doi:10.1002/etc.2042. ISSN 1552-8618. PMID 23109293.
- ↑ Zeng, Guangming; Zhang, Chang; Huang, Guohe; Yu, Jian; Wang, Qin; Li, Jianbing; Xi, Beidou; Liu, Hongliang (2006-11-01). "Adsorption behavior of bisphenol A on sediments in Xiangjiang River, Central-south China". Chemosphere. 65 (9): 1490–1499. Bibcode:2006Chmsp..65.1490Z. doi:10.1016/j.chemosphere.2006.04.013. PMID 16737729.
- ↑ Drewes, J. E.; Hemming, J.; Ladenburger, S. J.; Schauer, J.; Sonzogni, W. An assessment of endocrine disrupting activity changes during wastewater treatment through the use of bioassays and chemical measurements. Water Environ. Res. 2005, 77, 12–23.
- ↑ Klečka, G., Staples, C., Clark, K., Anderhoeven, N., Thomas, D. and Hentges, S. (2009). "Exposure analysis of Bisphenol A in surface water systems in North America and Europe". Environ. Sci. Technol. 43 (16): 6145–6150. Bibcode:2009EnST...43.6145K. doi:10.1021/es900598e.
- ↑ "Bisphenol A Fact Sheet". Government of Canada. Archived from the original on 23 April 2011. Retrieved 1 February 2012.
- ↑ Oehlmann J, Schulte-Oehlmann U, Kloas W, Jagnytsch O, Lutz I, Kusk KO, Wollenberger L, Santos EM, Paull GC, Van Look KJ, Tyler CR (2009). "A critical analysis of the biological impacts of plasticizers on wildlife". Philosophical Transactions of the Royal Society B: Biological Sciences. 364 (1526): 2047–62. doi:10.1098/rstb.2008.0242. PMC 2873012. PMID 19528055.
Further reading
- Kabiersch G, Rajasärkkä J, Ullrich R, Tuomela M, Hofrichter M, Virta M, Hatakka A, Steffen K (April 2011). "Fate of bisphenol A during treatment with the litter-decomposing fungi Stropharia rugosoannulata and Stropharia coronilla". Chemosphere. 83 (3): 226–32. Bibcode:2011Chmsp..83..226K. doi:10.1016/j.chemosphere.2010.12.094. PMID 21295326.
- Myers JP, vom Saal FS, Akingbemi BT, Arizono K, Belcher S, Colborn T, Chahoud I, Crain DA, Farabollini F, Guillette LJ, Hassold T, Ho SM, Hunt PA, Iguchi T, Jobling S, Kanno J, Laufer H, Marcus M, McLachlan JA, Nadal A, Oehlmann J, Olea N, Palanza P, Parmigiani S, Rubin BS, Schoenfelder G, Sonnenschein C, Soto AM, Talsness CE, Taylor JA, Vandenberg LN, Vandenbergh JG, Vogel S, Watson CS, Welshons WV, Zoeller RT (March 2009). "Why public health agencies cannot depend on good laboratory practices as a criterion for selecting data: the case of bisphenol A". Environ. Health Perspect. 117 (3): 309–15. doi:10.1289/ehp.0800173. PMC 2661896. PMID 19337501.