Alexander Dianin
| Alexander Pavlovich Dianin | |
|---|---|
 | |
| Born |
April 20, 1851 Davydovo, Vladimir Governorate, Russian Empire |
| Died |
December 6, 1918 (aged 67) Petrograd, RSFSR |
| Nationality | Russian |
| Alma mater |
University of Jena (PhD in Chemistry, 1877) Imperial Medical-Surgical Academy in St. Petersburg (MD, 1882) |
| Known for |
Bisphenol A Dianin's compound |
| Scientific career | |
| Fields | Organic chemistry |
| Institutions | Imperial Medical-Surgical Academy in St. Petersburg |
| Influences |
Alexander Borodin Nikolay Zinin |
| Influenced | Phoebus Levene[1] |
Alexander Pavlovich Dianin (Russian: Александр Павлович Дианин; April 20, 1851 – December 6, 1918) was a Russian chemist from Saint Petersburg. He carried out studies on phenols and discovered a phenol derivative now known as bisphenol A[2][3] and the accordingly named Dianin's compound.[4] He was married to the adopted daughter of fellow chemist Alexander Borodin. In 1887, Dianin succeeded his father-in-law as chair of the Chemistry Department at the Imperial Medical-Surgical Academy in St. Petersburg (now the S.M. Kirov Military Medical Academy).
Bisphenol A and Dianin's compound
Dianin's method for preparing bisphenol A from 1891[2] remains the most widely-known approach to this important compound,[5] though the method has been refined for industrial-scale synthesis.[6] It involves the catalysed condensation of a 2:1 mixture of phenol and acetone in the presence of concentrated hydrochloric acid or sulfuric acid. The reaction proceeds readily at room temperature producing a crude product containing a great variety of side products (including Dianin's compound) in a matter of hours.[5] The overall equation is simple, with water as the only by-product:
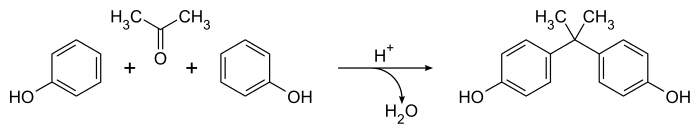
Mechanistically, the acid catalyst converts the acetone to a carbenium ion that undergoes an electrophilic aromatic substitution reaction with the phenol, producing predominantly para-substituted products. A second carbenium species is produced by protonation and loss of the aliphatic hydroxyl group, leading to bisphenol A (4,4'-isopropylidenediphenol) after a second aromatic substitution reaction. The process is not very selective, and a great number of minor products and side reactions are known.[5]
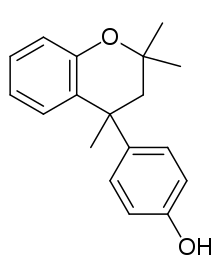
Side products that are isomers of bisphenol A result from the formation of ortho-substituted products, and include the 2,2'- and 2,4'- isomers of isopropylidenediphenol. Other side reactions include the formation of triphenol I, 4,4'-(4-hydroxy-m-phenylenediisopropylidene)diphenol, from the attack of a carbenium electrophile on a bispheol A molecule and the formation of triphenol II, 4,4',4''-(2-methyl-2-pentanyl-4-ylidene)triphenol, when an elimination reaction converts the carbenium to a reactive olefin.[5] Catalysed dimerisation of acetone via an aldol condensation is well known, and yields diacetone alcohol and (by dehydration) mesityl oxide in both acidic[7] and basic conditions.[8][9] The in situ generation of mesityl oxide adds another reactive olefin to the mixture. In cases where an olefinic moiety can interact with a phenolic hydoxyl group (tyupically as a result of ortho-substitution), rapid cyclisation reactions producing flavans and chromans occur.[5] This is the source of Dianin's compound in the mixture, and Dianin subsequently demonstrated that the compound can be produced in much greater yield by reacting phenol with mesityl oxide directly.[4] Later work has shown that production of bisphenol A can be made much more selective by using a reaction mixture with a considerable excess of phenol rather than a stoichiometric 2:1 composition, greatly suppressing side reactions.[6]
Further reading
- Dianin, Sergey Aleksandrovich (1980). Borodin. Westport: Greenwood Press. ISBN 9780313225291. OCLC 247826062.
- Figurovskiy, Nikolay Aleksandrovich; Soloviev, Yuriy Ivanovich (1988). Aleksandr Porfirievich Borodin: a chemist's biography. New York: Springer-Verlag. p. 22. ISBN 9780387178882. OCLC 16647830.
References
- ↑ Tipson, R. Stuart (1957). Wolfrom, M. L., ed. Phoebus Aaron Theodore Levene: 1869–1940. Obituary. Advances in Carbohydrate Chemistry. 12. New York: Academic Press. pp. 1–12. ISBN 9780080562711.
- 1 2 Dianin, A. P. (1891). "О продуктах конденсации кетонов с фенолами" [Condensation of ketones with phenols]. Журнал Русского Физико-Химического Общества (J. Russ. Phys. Chem. Soc.) (in Russian). 23: 488–517, 523–546, 601–611.
- ↑ Zincke, Theodor (1905). "Ueber die Einwirkung von Brom und von Chlor auf Phenole: Substitutionsprodukte, Pseudobromide und Pseudochloride" [On the effect of bromine and chlorine on phenols: Substitution products, pseudobromides and pseudochlorides]. Justus Liebigs Annalen der Chemie (in German). 343: 75–99. doi:10.1002/jlac.19053430106.
- 1 2 Dianin, A. P. (1914). "Condensation of phenol with unsaturated ketones. Condensation of phenol with mesityl oxide". Журнал Русского Физико-Химического Общества (J. Russ. Phys. Chem. Soc.) (in Russian). 36: 1310–1319.
- 1 2 3 4 5 McKetta, John J., ed. (1977). "Bisphenol A". Asphalt Emulsion to Blending. Encyclopedia of Chemical Processing and Design. 4. Marcel Dekker. pp. 406–430. ISBN 9780824724542.
- 1 2 Fiege, Helmut; Voges, Heinz-Werner; Hamamoto, Toshikazu; Umemura, Sumio; Iwata, Tadao; Miki, Hisaya; Fujita, Yasuhiro; Buysch, Hans-Josef; Garbe, Dorothea; Paulus, Wilfried (2000). "Phenol Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a19_313.
- ↑ Weissermel, Klaus; Arpe, Hans-Jürgen (2003). "Secondary Products of Acetone". Industrial Organic Chemistry (4th ed.). John Wiley & Sons. pp. 281–288. ISBN 9783527305780.
- ↑ Conant, James B.; Tuttle, Neal (1921). "Diacetone alcohol (2-pentane, 4-hydroxy-4-methyl)". Org. Synth. 1: 45. doi:10.15227/orgsyn.001.0045. ; Coll. Vol., 1, p. 199
- ↑ Conant, James B.; Tuttle, Neal (1921). "Mesityl Oxide". Org. Synth. 1: 53. doi:10.15227/orgsyn.001.0053. ; Coll. Vol., 1, p. 345