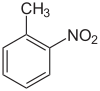
2-Nitrotoluene
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1-methyl-2-nitro-benzene | |||
| Other names
o-Nitrotoluene, o-Methylnitrobenzene, 2-Methylnitrobenzene, ortho-Nitrotoluene | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.685 | ||
| |||
| |||
| Properties | |||
| C7H7NO2 | |||
| Molar mass | 137.14 g·mol−1 | ||
| Appearance | yellow liquid[1] | ||
| Odor | weak, aromatic[1] | ||
| Density | 1.1611 g·cm−3 @ 19°C [2] | ||
| Melting point | −10.4 °C (13.3 °F; 262.8 K)[2] | ||
| Boiling point | 222 °C (432 °F; 495 K)[2] | ||
| 0.07% (20°C)[1] | |||
| Vapor pressure | 0.1 mmHg (20°C)[1] | ||
| -72.28·10−6 cm3/mol | |||
| Hazards | |||
| Flash point | 106 °C; 223 °F; 379 K [1] | ||
| Explosive limits | 2.2%-?[1] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
891 mg/kg (oral, rat) 970 mg/kg (oral, mouse) 1750 mg/kg (oral, rabbit)[3] | ||
| US health exposure limits (NIOSH): | |||
PEL (Permissible) |
TWA 5 ppm (30 mg/m3) [skin][1] | ||
REL (Recommended) |
TWA 2 ppm (11 mg/m3) [skin][1] | ||
IDLH (Immediate danger) |
200 ppm[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
2-Nitrotoluene or ortho-nitrotoluene is an organic compound with the formula CH3C6H4NO2. It is pale yellow liquid that is a versatile intermediate in the production of various dyes.[4]
Synthesis and reactions
It is made by nitrating toluene at above -10 °C. This reaction affords a 2:1 mixture of 2-nitro and 4-nitro isomers.[4]
Chlorination gives two isomers of the chloronitrotoluenes. Similarly nitration gives two isomers of dinitrotoluene.
2-Nitrotolune is mainly consumed in the production of o-toluidine, a precursor to dyes.[4]
References
- 1 2 3 4 5 6 7 8 9 "NIOSH Pocket Guide to Chemical Hazards #0462". National Institute for Occupational Safety and Health (NIOSH).
- 1 2 3 Lide DR, ed. (2004). CRC handbook of chemistry and physics: a ready-reference book of chemical and physical data (85 ed.). Boca Ratan Florida: CRC Press. ISBN 0-8493-0485-7.
- ↑ "Nitrotoluene (o-, m-, p-isomers)". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- 1 2 3 Gerald Booth (2007). "Nitro Compounds, Aromatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_411.
External links
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.