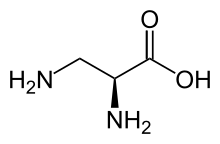
2,3-Diaminopropionic acid
 | |
| Names | |
|---|---|
| IUPAC name
(2S)-2,3-Diaminopropanoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C3H8N2O2 | |
| Molar mass | 104.11 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2,3-Diaminopropionic acid (2,3-diaminopropionate) is a non-proteinogenic amino acid found in certain secondary metabolites, including zwittermicin A[1] and tuberactinomycin.[2]
Biosynthesis
2,3-Diaminopropionate is formed by the pyridoxal phosphate (PLP) mediated amination of serine.
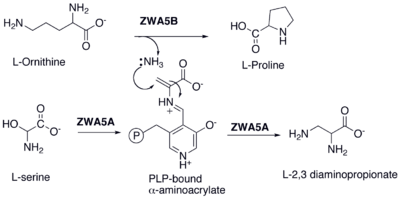
Biosynthesis of L-2,3 Diaminopropionate
References
- ↑ Rogers EW, Molinski TF (February 2007). "Asymmetric synthesis of diastereomeric diaminoheptanetetraols. A proposal for the configuration of (+)-zwittermicin a". Org. Lett. 9 (3): 437–40. doi:10.1021/ol062804a. PMC 2729442. PMID 17249781.
- ↑ Michael G. Thomas (2003). "ADeciphering Tuberactinomycin Biosynthesis: Isolation, Sequencing, and Annotation of the Viomycin Biosynthetic Gene Cluster". Antimicrob. Agents Chemother. 47 (9): 2823–2830. doi:10.1128/AAC.47.9.2823-2830.2003. PMC 182626. PMID 12936980.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.