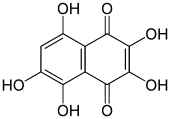
2,3,5,6,8-Pentahydroxy-1,4-naphthalenedione
 | |
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C10H6O7 | |
| Molar mass | 238.15 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2,3,5,6,8-Pentahydroxy-1,4-naphthalenedione, also called 2,3,5,6,8-pentahydroxy-1,4-naphthoquinone or spinochrome D, is an organic compound with formula C
10H
6O
5, formally derived from 1,4-naphthoquinone through the replacement of five hydrogen atoms by hydroxyl (OH) groups.
Spinochrome D occurs naturally as a brownish red pigment in the shell and spines of sea urchins such as the Japanese aka-uni (Pseudocentrotus depressus).[1] It is soluble in diethyl ether and crystallizes as brownish red needles that sublime at 285−295 °C.[1]
The compound gives a yellowish brown solution when treated with sodium hydroxide, a bluish green solution with ferric chloride, and a violet precipitate with lead acetate. It forms a five-fold acetate ester, C
10HO
2(CH
3COO)5, that crystallizes from methanol as yellow needles that melt at 185−186 °C.[1]
See also
- Hexahydroxynaphthoquinone (spinochrome E)
- 2,3,5,7-Tetrahydroxy-1,4-naphthoquinone (spinochrome B)
References
- 1 2 3 Chika KURODA and Masae OKAJIMA (1967), Studies on the Derivatives of Naphthoquinones, XVIII. The pigments of sea urchins, XIII. Proc. Japan Acad., volume 43, pages 41--44. Online version accessed on 2010-02-01.