1,2-Dioxin
 | |||
| |||
| Names | |||
|---|---|---|---|
| Systematic IUPAC name
1,2-Dioxine[1] | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
PubChem CID |
|||
| |||
| |||
| Properties | |||
| C4H4O2 | |||
| Molar mass | 84.07 g·mol−1 | ||
| Related compounds | |||
Related compounds |
Dibenzodioxin | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
1,2-Dioxin is a heterocyclic, organic, antiaromatic compound with the chemical formula C4H4O2. It is an isomeric form of 1,4-dioxin (or p-dioxin).
Due to its peroxide-like characteristics, 1,2-dioxin is very unstable and has not been isolated. Even substituted derivatives are very labile, e.g. 1,4-diphenyl-2,3-benzodioxin.[2] Indeed, in 1990, 3,6-bis(p-tolyl)-1,2-dioxin was wrongly accounted as the first stable derivative.[3] It was subsequently shown that the initial compound was not a derivative of 1,2-dioxin, but a thermodynamically more stable dione.[4]
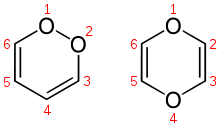 The isomers 1,2-dioxin (left) and 1,4-dioxin (right)
The isomers 1,2-dioxin (left) and 1,4-dioxin (right)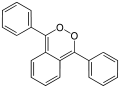 Structure of the transient 1,4-diphenyl- 2,3-benzodioxin
Structure of the transient 1,4-diphenyl- 2,3-benzodioxin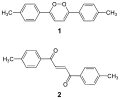 Dioxin (1) and dione form (2)
Dioxin (1) and dione form (2)
References
- ↑ "CID 15559065 - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 12 February 2002. Identification and Related Records. Retrieved 7 October 2011.
- ↑ Smith, Jimmie P.; Schrock, Alan K.; Schuster, Gary B. (1982). "Chemiluminescence of organic peroxides. Thermal generation of an o-xylylene peroxide". Journal of the American Chemical Society. 104 (4): 1041. doi:10.1021/ja00368a021. .
- ↑ Shine, Henry J.; Zhao, Da Chuan (1990). "Electron transfer to excited doublet states. Photoirradiation of 10-methylphenothiazine cation radical perchlorate in solutions of phenylacetylene and p-tolylacetylene in acetonitrile". The Journal of Organic Chemistry. 55 (13): 4086. doi:10.1021/jo00300a026. .
- ↑ Block, Eric; Shan, Zhixing; Glass, Richard S.; Fabian, Jürgen (2003). "Revised Structure of a Purported 1,2-Dioxin: A Combined Experimental and Theoretical Study". The Journal of Organic Chemistry. 68 (10): 4108. doi:10.1021/jo034305i. PMID 12737603.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.