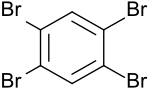
1,2,4,5-Tetrabromobenzene
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ECHA InfoCard | 100.010.231 |
| EC Number | 211-253-3 |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C6H2Br4 | |
| Hazards | |
| GHS pictograms |  |
| GHS signal word | Warning |
| H315, H319, H335, H413 | |
| P261, P264, P271, P273, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
1,2,4,5-Tetrabromobenzene is a fourfold symmetrically bromine-substituted benzene and starting material for liquid crystals and OLED materials, as well as for mono-[1] and bis-aryines.[2] 1,2,4,5-Tetrabromobenzene is an important metabolite of the completely brominated hexabromobenzene used as a flame retardant in the animal organism with liver-damaging properties.[3]
Preparation
The synthesis of 1,2,4,5-tetrabromobenzene has already been reported in 1865 from benzene and excess bromine in a sealed tube at 150 °C.[4] However, the clearly reduced melting point of about 160 °C indicates impurities in the final product.
In 1885, Adolf Scheufelen published the synthesis of 1,2,4,5-tetrabromobenzene in the presence of ion(III) chloride FeCl3 as a catalyst in his dissertation and obtained a purer product (mp 175 °C) in "pretty needles" ("schönen Nadeln").[5]
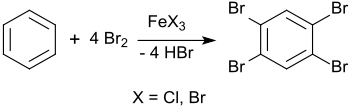
The synthesis can also be carried out in solution in chloroform or tetrachloromethane and yields 1,2,4,5-tetrabromobenzene in 89% yield.[6]
As a teaching example for electrophilic aromatic substitutions, this reaction can also be carried out in a laboratory experiment with excess bromine and iron nails (as starting material for iron (III) bromide FeBr3).[7] The intermediate stage is 1,4-dibromobenzene, which reacts further with excess bromine to give 1,2,4,5-tetrabromobenzene.
Use
Building block for liquid crystals and fluorescent dyes
The symmetrical substitution pattern with reactive bromine atoms makes 1,2,4,5-tetrabromobenzene an interesting starting compound for nematic liquid crystals[8] with crossed mesogens
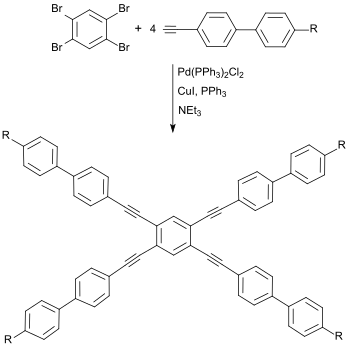
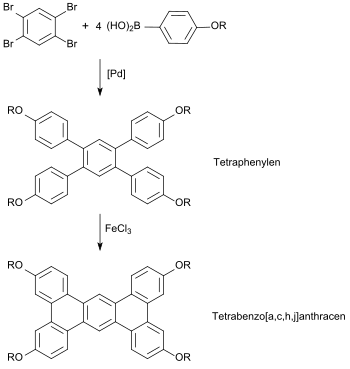
and for columnar (diskotic) liquid crystals[9][10] with an extensive planar, "board-like" tetrabenzoanthracene ring system.
In a one-pot reaction, 1,2,4,5-tetrabromobenzene can be reacted with the aromatic aldehyde 4-hydroxybenzaldehyde, the alkylating agent 1-bromopentane, the Wittig reagent methyltriphenylphosphonium iodide, the base potassium carbonate, the phase transfer catalyst tetrabutylammonium bromide, the Heck reagent palladium(II)acetate and the Heck co-catalyst 1,3-bis(diphenylphosphino)propane (dppp) in dimethylacetamide obtaining directly a symmetrical tetraalkoxylstilbene as E-isomer in 17% yield.[11]
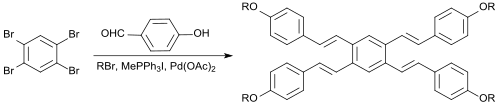
Due to their pronounced π-conjugation such compounds could be potentially applied as optical brighteners, OLED materials or liquid crystals.
N-alkyl-tetraaminobenzenes are available from 1,2,4,5-tetrabromobenzene in high yields, which can be cyclized with triethyl orthoformate and acids to benzobis(imidazolium) salts (BBI salts) and oxidized with oxygen to form 1,4-benzoquinone diimines.[12]
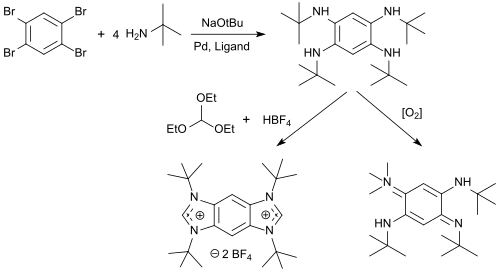
BBI salts are versatile fluorescent dyes with emission wavelengths λem between 329 and 561 nm, pronounced solvatochromism and strong solvent-dependent Stokes shift, which can be used as protein tag for fluorescent labeling of proteins.[13]
Starting material for arines
From 1,2,4,5-tetrabromobenzene, a 1,4-monoarine can be prepared in-situ with one equivalent of n-butyllithium by bromine abstraction, which reacts immediately with furan to form 6,7-dibromo-1,4-epoxy-1,4-dihydronaphthalene (6,7-dibromonaphthalene-1,4-endoxide) in 70% yield.[1]
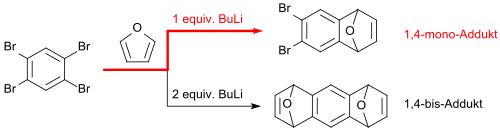
When 2,5-dialkylfurans (e.g. 2,5- (di-n-octyl)furan) are used, the dibrominated monoendoxide is formed in 64% yield, from which dibromo-5,8-di-n-octylnaphthalene is formed with zink powder/titanium tetrachloride in 88% yield.[14]
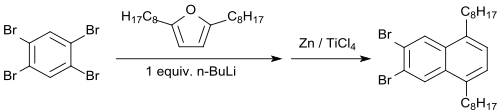
With titanium tetrachloride/zinc powder, the endoxide can be reduced to the 2,3-dibromnaphthalene in 86% yield.[15]

The endoxide reacts with 3-sulfolene in a Diels-Alder reaction upon elimination of sulfur dioxide to form a tricyclic adduct, from which 2,3-dibromoanthracene is accessible in good yield.[16]
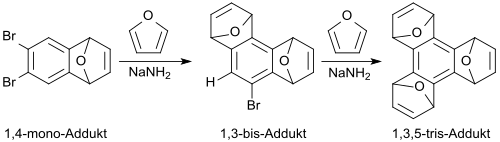
If the dibromene oxide is allowed to react further with furan, in the presence of n-butyllithium[1] or potassium amide[17] or via an intermediate 1,4-aryne the tricyclic 1,4-adduct 1,4:5.8-diepoxy-1,4,5,8-tetrahydroanthracene[18] is formed in 71% yield as a syn-anti-mixture.
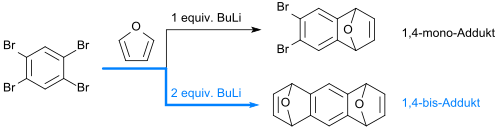
With sodium amide in ethylene glycol dimethyl ether (DME), however, the dibromene oxide behaves as a 1,3-aryene equivalent and forms with furan a phenanthrene-like tricyclic 1,3-adduct, which can react with furan and sodium amide to a triphenylene derivative (1,3,5-tris-arene).[17]
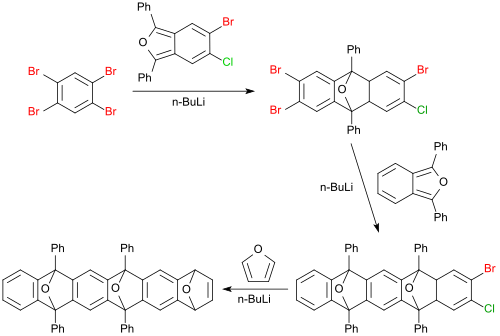
[2+4] cycloadditions with 1,2,4,5-tetrabromobenzene sometimes proceed in very high yields, such as the reaction of a dihalogen-substituted 1,3-diphenyl-isobenzofuran to a tetrahalogenated anthracene derivative (98%), which is converted successively further with 1,3-diphenyl isobenzofuran in 65% yield to a pentacene derivative and furan to a hexacene derivative (67%).[19]
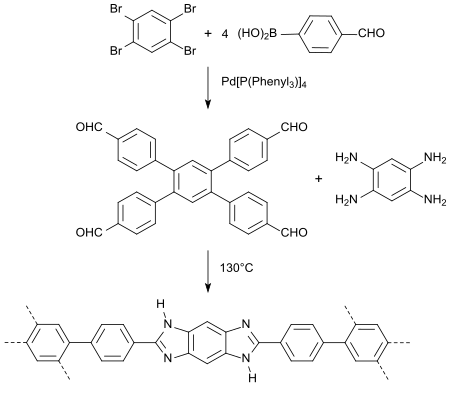
The crosslinking of benzimidazole-modified polymers provides materials with a high absorption capacity for carbon dioxide, which could be suitable for CO2 separation from gas mixtures.[20]
Safety
1,2,4,5-Tetrabromobenzene is a liver toxic degradation product of the flame retardant hexabromobenzene and was already in 1987 detected in Japan in mother's milk samples.[21]
References
- 1 2 3 H. Hart, A. Bashir-Hashemi, J. Luo, M.A. Meador (1986), "Iptycenes: Extended triptycenes", Tetrahedron 42 (6): pp. 1641–1654, doi:10.1016/S0040-4020(01)87581-5
- ↑ "USE OF 1,2,4,5-TETRABROMOBENZENE AS A 1,4-BENZADIYNE EQUIVALENT: anti- AND syn-1,4,5,8-TETRAHYDROANTHRACENE 1,4:5,8-DIEPOXIDES". Organic Syntheses. doi:10.15227/orgsyn.075.0201.
- ↑ E. Bruchajzer, B. Frydrych, J.A. Szymanska (2004), "Effect of repeated administration of hexabromobenzene and 1,2,4,5-tetrabromobenzene on the levels of selected cytochromes in rat liver", Int. J. Occup. Med. Environ. Health 17 (3): pp. 347–353, doi:10.1016/S0040-4020(01)87581-5
- ↑ A. Riche, P. Bérard (1865), "Ueber die bromhaltigen Derivate des Benzols und seiner Homologen" (in German), Liebigs Ann. Chem. 133 (1): pp. 51–54, doi:10.1002/jlac.18651330106
- ↑ A. Scheufelen (1885), "Ueber Eisenverbindungen als Bromüberträger" (in German), Liebigs Ann. Chem. 231 (2): pp. 152–195, doi:10.1002/jlac.18852310204
- ↑ US 0
- ↑ B. Cox, D.G. Kubler, C.A. Wilson (1977), "Experiments with electrophilic aromatic substitution reactions", J. Chem. Educ. 54 (6): p. 379, doi:10.1021/ed054p379
- ↑ H.-H. Chen et al. (2012), "Enantiotropic nematics from cross-like 1,2,4,5-tetrakis(4’-alkyl-4-ethynylbiphenyl) benzenes and their biaxiality studies", Chem. Eur. J. 18 (31): pp. 9543–9551, doi:10.1002/chem.201103453
- ↑ S. Kumar (2011), Chemistry of discotic liquid crystals: from monomers to polymers, Boca Raton, FL, U.S.A.: CRC Press, p. 200, ISBN 978-1-4398-1145-0
- ↑ M.C. Artal, K.J. Toyne, J.W. Goodby, J. Barbera, D.J. Photinos (2011), "Synthesis and mesogenic properties of novel board-like liquid crystals", J. Mater. Chem. 11: pp. 2801–2807, doi:10.1039/B105351P
- ↑ K.N. Patel, B.V. Kamath, A.V. Bedekar (2013), "Synthesis of alkyloxy stilbenes by one-pot O-alkylation-Wittig and O-alkylation-Wittig-Heck reaction sequence", Tetrahedron Lett. 54 (1): pp. 80–84, doi:10.1016/tetlet.2012.10.102
- ↑ D.M. Khramov, A.J. Boydston, C.W. Bielawski (2006), "Highly efficient synthesis and solid-state characterization of 1,2,4,5-tetrakis(alkyl- and arylamino)benzenes and cyclization to their respective benzobis(imidazolium) salts", Org. Lett. 8 (9): pp. 1831–1834, doi:10.1021/ol060349c
- ↑ A.J. Boydston (2008), "Modular fluorescent benzobis(imidazolium)saltes: Syntheses, photophysical analyses, and applications", J. Am. Chem. Soc. 130 (10): pp. 3143–3156, doi:10.1021/ja7102247
- ↑ Z. Chen, P. Müller, T.M. Swager (2006), "Syntheses of soluble, π-stacking tetracene derivatives", Org. Lett. 8 (2): pp. 273–276, doi:10.1021/ol0526468
- ↑ H. Hart, C.-Y. Lai, G.C. Nwokogu, S. Shamouilian (1987), "Tetrahalobenzenes as diaryne equivalents in polycyclic arene synthesis", Tetrahedron 43 (22): pp. 5203–5224, doi:10.1016/S0040-4020(01)87696-1
- ↑ C.-T. Lin, T.-C. Chou (1988), "Synthesis of 2,3-dibromoanthracene", Synthesis 1988 (8): pp. 628–630, doi:10.1055/s-1988-27659
- 1 2 F. Raymo, F.H. Kohnke, F. Cardullo (1992), "The regioselective generation of arynes from polyhalogenobenzenes. An improved synthesis of syn- and anti-1,4,5,8,9,12-hexahydro-1,4:5,8:9,12-triepoxytriphenylene" (in German), Tetrahedron 48 (33): pp. 6827–6838, doi:10.1016/S0040-4020(01)89874-4
- ↑ H. Hart, N. Raju, M.A. Meador, D.L. Ward (1983), "Synthesis of heptiptycenes with face-to-face arene rings via a 2,3:6,7-anthradiyne equivalent", J. Org. Chem. 48 (23): pp. 4357–4360, doi:10.1021/jo00171a039
- ↑ S. Eda, T. Hamura (2015), "Selective Halogen-Lithium Exchange of 1,2-Dihaloarenes for Successive [2+4] Cycloadditions of Arynes and Isobenzofurans", Molecules 20: pp. 19449–19462, doi:10.3390/molecules201019449
- ↑ S. Altarawneh, S. Behera, P. Jena, H.M. El-Kaderi (2014), "New insights into carbon dioxide interactions with benzimidazole-linked polymers", Chem. Commun. 50: pp. 3571–3574, doi:10.1039/C3CC45901B
- ↑ T. Miyazaki, T. Yamagishi, M. Matsumoto (1987), "Determination and residual levels of 1,2,4,5-tetrabromobenzene and Mirex in human milk samples", Food Hygiene and Safety Science 28 (2): pp. 125–129, doi:10.3358/shokueishi.28.125