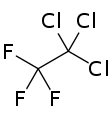
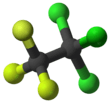
1,1,1-Trichloro-2,2,2-trifluoroethane
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1,1,1-Trichloro-2,2,2-trifluoroethane | |||
| Other names
CFC-113a Freon 113a Arcton 63 Freon-FT 1,1,1-Trichloro-2,2,2-trifluoroethane 1,1,1-Trichlorotrifluoroethane 1,1,1-Trifluoro-2,2,2-trichloroethane 1,1,1-Trifluorotrichloroethane CF3CCl3 FC 113 FC133a Precision cleaning agent TF T-WD602 Trichlorotrifluoroethane FC 113a 2,2,2-Trichloro-1,1,1-trifluoro-ethane | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.005.968 | ||
| EC Number | 206-564-6 | ||
PubChem CID |
|||
| |||
| |||
| Properties | |||
| C2Cl3F3 | |||
| Molar mass | 187.376 g/mol | ||
| Density | 1.579 g/mL[1][2] | ||
| Melting point | 13–14 °C (55–57 °F; 286–287 K) | ||
| Boiling point | 46 °C (115 °F; 319 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Trichlorotrifluoroethane, also called 1,1,1-Trichloro-2,2,2-trifluoroethane or CFC-113a is a chlorofluorocarbon (CFC). It has the formula Cl3C-CF3.
Environmental effects
Ozone depletion
It is one of four man-made chemicals newly discovered in the atmosphere by a team at the University of East Anglia. But CFC-113a is the only known CFC whose abundance in the atmosphere is still growing. CFC-113a seems to have been accumulating unabated since 1960. Its source remains uncertain, but production of hydrofluorocarbons in East Asia is suspected by some. Between 2012 and 2017, concentrations of the gas jumped by 40 percent.[3][4][5]
See also
References
- ↑ "1,1,1-Trichlorotrifluoroethane". chemblink.com. Retrieved 10 March 2014.
- ↑ "Material Safety Data Sheet : 1,1,1-Trichlorotrifluoroethane". fishersci.com. Retrieved 10 March 2014.
- ↑ Adcock, Karina; Reeves, Claire; Gooch, Lauren; Leedham Elvidge, Emma; Ashfold, Matthew; Brenninkmeijer, Carl; Chou, Charles; Fraser, Paul; Langenfelds, Ray; Mohd Hanif, Norfazrin; O'Doherty, Simon; Oram, David; Ou-Yang, Chang-Feng; Phang, Siew Moi; Samah, Azizan Abu; Röckmann, Thomas; Sturges, William; Laube, Johannes (9 April 2018). "Continued increase of CFC-113a (CCl3CF3) mixing ratios in the global atmosphere: emissions, occurrence and potential sources". Atmospheric Chemistry and Physics. doi:10.5194/acp-18-4737-2018. Retrieved 17 April 2018.
- ↑ Laube, Johannes C.; Newland, Mike J.; Hogan, Christopher; Brenninkmeijer, Carl A. M.; Fraser, Paul J.; Martinerie, Patricia; Oram, David E.; Reeves, Claire E.; Röckmann, Thomas; Schwander, Jakob; Witrant, Emmanuel; Sturges, William T. (9 March 2014). "Newly detected ozone-depleting substances in the atmosphere". Nature Geoscience. doi:10.1038/ngeo2109. Retrieved 10 March 2014.
- ↑ McGrath, Matt. "Mysterious new man-made gases pose threat to ozone layer". BBC. Retrieved 10 March 2014.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.