Phenotypic plasticity
Phenotypic plasticity refers to some of the changes in an organism's behavior, morphology and physiology in response to a unique environment.[1] Fundamental to the way in which organisms cope with environmental variation, phenotypic plasticity encompasses all types of environmentally induced changes (e.g. morphological, physiological, behavioural, phenological) that may or may not be permanent throughout an individual's lifespan. The term was originally used to describe developmental effects on morphological characters, but is now more broadly used to describe all phenotypic responses to environmental change, such as acclimation (acclimatization), as well as learning.[2] The special case when differences in environment induce discrete phenotypes is termed polyphenism.
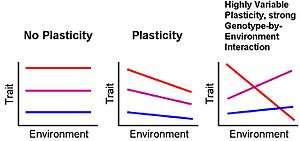
Generally, phenotypic plasticity is more important for immobile organisms (e.g. plants) than mobile organisms (e.g. most animals), as mobile organisms can often move away from unfavourable environments.[3] Nevertheless, mobile organisms also have at least some degree of plasticity in at least some aspects of the phenotype. One mobile organism with substantial phenotypic plasticity is Acyrthosiphon pisum of the aphid family, which exhibits the ability to interchange between asexual and sexual reproduction, as well as growing wings between generations when plants become too populated.[4]
Examples
Plants

Phenotypic plasticity in plants includes the timing of transition from vegetative to reproductive growth stage, the allocation of more resources to the roots in soils that contain low concentrations of nutrients, the size of the seeds an individual produces depending on the environment,[5] and the alteration of leaf shape, size, and thickness.[6] Leaves are particularly plastic, and their growth may be altered by light levels. Leaves grown in the light tend to be thicker, which maximizes photosynthesis in direct light; and have a smaller area, which cools the leaf more rapidly (due to a thinner boundary layer). Conversely, leaves grown in the shade tend to be thinner, with a greater surface area to capture more of the limited light.[7][8] Dandelion are well known for exhibiting considerable plasticity in form when growing in sunny versus shaded environments. The transport proteins present in roots also change depending on the concentration of the nutrient and the salinity of the soil.[9] Some plants, Mesembryanthemum crystallinum for example, are able to alter their photosynthetic pathways to use less water when they become water- or salt-stressed.[10]
Because of phenotypic plasticity, it is hard to explain and predict the traits when plants are grown in natural conditions unless an explicit environment index can be obtained to quantify environments. Identification of photothermal time from a critical growth periods being highly correlated with sorghum flowering time enables such predictions.[11]
Phytohormones and leaf plasticity
Leaves are very important to a plant in that they create an avenue where photosynthesis and thermoregulation can occur. Evolutionarily, the environmental contribution to leaf shape allowed for a myriad of different types of leaves to be created.[12] Leaf shape can be determined by both genetics and the environment [13]. Environmental factors, such as light and humidity, have been shown to affect leaf morphology,[14] giving rise to the question of how this shape change is controlled at the molecular level. This means that different leaves could have the same gene but present a different form based on environmental factors. Plants are sessile, so this phenotypic plasticity allows the plant to take in information from its environment and respond without changing its location.
In order to understand how leaf morphology works, the anatomy of a leaf must be understood. The main part of the leaf, the blade or lamina, consists of the epidermis, mesophyll, and vascular tissue. The epidermis contains stomata which allows for gas exchange and controls perspiration of the plant. The mesophyll contains most of the chloroplast where photosynthesis can occur. Developing a wide blade/lamina can maximize the amount of light hitting the leaf, thereby increasing photosynthesis, however too much sunlight can damage the plant. Wide lamina can also catch wind easily which can cause stress to the plant, so finding a happy medium is imperative to the plants’ fitness. The Genetic Regulatory Network is responsible for creating this phenotypic plasticity and involves a variety of genes and proteins regulating leaf morphology. Phytohormones have been shown to play a key role in signaling throughout the plant, and changes in concentration of the phytohormones can cause a change in development.[15]
Studies on the aquatic plant species Ludwigia arcuata have been done to look at the role of abscisic acid (ABA), as L. arcuata is known to exhibit phenotypic plasticity and has two different types of leaves, the aerial type (leaves that touch the air) and the submerged type (leaves that are underwater).[16] When adding ABA to the underwater shoots of L. arcuata, the plant was able to produce aerial type leaves underwater, suggesting that increased concentrations of ABA in the shoots, likely caused by air contact or a lack of water, triggers the change from the submerged type of leaf to the aerial type. This suggests ABA's role in leaf phenotypic change and its importance in regulating stress through environmental change (such as adapting from being underwater to above water). In the same study, another phytohormone, ethylene, was shown to induce the submerged leaf phenotype unlike ABA, which induced aerial leaf phenotype. Because ethylene is a gas, it tends to stay endogenously within the plant when underwater – this growth in concentration of ethylene induces a change from aerial to submerged leaves and has also been shown to inhibit ABA production, further increasing the growth of submerged type leaves. These factors (temperature, water availability, and phytohormones) contribute to changes in leaf morphology throughout a plants lifetime and are vital to maximize plant fitness.
Animals
The developmental effects of nutrition and temperature have been demonstrated.[17] The gray wolf (Canis lupus) has wide phenotypic plasticity.[18][19] Additionally, male speckled wood butterflies have two morphs: one with three dots on its hindwing, and one with four dots on its hindwings. The development of the fourth dot is dependent on environmental conditions – more specifically, location and the time of year.[20] In amphibians, Pristimantis mutabilis has remarkable phenotypic plasticity.[21] Another example is the southern rockhopper penguin.[22] Rockhopper penguins are present at a variety of climates and locations; Amsterdam Island's subtropical waters, Kerguelen Archipelago's subarctic coastal waters, and Crozet Archipelago's subantarctic coastal waters.[22] Due to the species plasticity they are able to express different strategies and foraging behaviors depending on the climate and environment.[22] A main factor that has influenced the species' behavior is where food is located.[22]
Temperature
Plastic responses to temperature are essential among ectothermic organisms, as all aspects of their physiology are directly dependent on their thermal environment. As such, thermal acclimation entails phenotypic adjustments that are found commonly across taxa, such as changes in the lipid composition of cell membranes. Temperature change influences the fluidity of cell membranes by affecting the motion of the fatty acyl chains of glycerophospholipids. Because maintaining membrane fluidity is critical for cell function, ectotherms adjust the phospholipid composition of their cell membranes such that the strength of van der Waals forces within the membrane is changed, thereby maintaining fluidity across temperatures.[23]
Diet
Phenotypic plasticity of the digestive system allows some animals to respond to changes in dietary nutrient composition,[24][25] diet quality,[26][27] and energy requirements.[28][29][30]
Changes in the nutrient composition of the diet (the proportion of lipids, proteins and carbohydrates) may occur during development (e.g. weaning) or with seasonal changes in the abundance of different food types. These diet changes can elicit plasticity in the activity of particular digestive enzymes on the brush border of the small intestine. For example, in the first few days after hatching, nestling house sparrows (Passer domesticus) transition from an insect diet, high in protein and lipids, to a seed based diet that contains mostly carbohydrates; this diet change is accompanied by two-fold increase in the activity of the enzyme maltase, which digests carbohydrates.[24] Acclimatizing animals to high protein diets can increase the activity of aminopeptidase-N, which digests proteins.[25][31]
Poor quality diets (those that contain a large amount of non-digestible material) have lower concentrations of nutrients, so animals must process a greater total volume of poor-quality food to extract the same amount of energy as they would from a high-quality diet. Many species respond to poor quality diets by increasing their food intake, enlarging digestive organs, and increasing the capacity of the digestive tract (e.g. prairie voles,[30] Mongolian gerbils,[27] Japanese quail,[26] wood ducks,[32] mallards[33]). Poor quality diets also result in lower concentrations of nutrients in the lumen of the intestine, which can cause a decrease in the activity of several digestive enzymes.[27]
Animals often consume more food during periods of high energy demand (e.g. lactation or cold exposure in endotherms), this is facilitated by an increase in digestive organ size and capacity, which is similar to the phenotype produced by poor quality diets. During lactation, common degus (Octodon degus) increase the mass of their liver, small intestine, large intestine and cecum by 15–35%.[28] Increases in food intake do not cause changes in the activity of digestive enzymes because nutrient concentrations in the intestinal lumen are determined by food quality and remain unaffected.[28] Intermittent feeding also represents a temporal increase in food intake and can induce dramatic changes in the size of the gut; the Burmese python (Python molurus bivittatus) can triple the size of its small intestine just a few days after feeding.[34]
AMY2B (Alpha-Amylase 2B) is a gene that codes a protein that assists with the first step in the digestion of dietary starch and glycogen. An expansion of this gene in dogs would enable early dogs to exploit a starch-rich diet as they fed on refuse from agriculture. Data indicated that the wolves and dingo had just two copies of the gene and the Siberian Husky that is associated with hunter-gatherers had just three or four copies, whereas the Saluki that is associated with the Fertile Crescent where agriculture originated had 29 copies. The results show that on average, modern dogs have a high copy number of the gene, whereas wolves and dingoes do not. The high copy number of AMY2B variants likely already existed as a standing variation in early domestic dogs, but expanded more recently with the development of large agriculturally based civilizations.[35]
Parasitism
Infection with parasites can induce phenotypic plasticity as a means to compensate for the detrimental effects caused by parasitism. Commonly, invertebrates respond to parasitic castration or increased parasite virulence with fecundity compensation in order to increase their reproductive output, or fitness. For example, water fleas (Daphnia magna), exposed to microsporidian parasites produce more offspring in the early stages of exposure to compensate for future loss of reproductive success.[36] A reduction in fecundity may also occur as a means of re-directing nutrients to an immune response,[37] or to increase longevity of the host.[38] This particular form of plasticity has been shown in certain cases to be mediated by host-derived molecules (e.g. schistosomin in snails Lymnaea stagnalis infected with trematodes Trichobilharzia ocellata) that interfere with the action of reproductive hormones on their target organs.[39] Changes in reproductive effort during infection is also thought to be a less costly alternative to mounting resistance or defence against invading parasites, although it can occur in concert with a defence response.[40]
Hosts can also respond to parasitism through plasticity in physiology aside from reproduction. House mice infected with intestinal nematodes experience decreased rates of glucose transport in the intestine. To compensate for this, mice increase the total mass of mucosal cells, cells responsible for glucose transport, in the intestine. This allows infected mice to maintain the same capacity for glucose uptake and body size as uninfected mice.[41]
Phenotypic plasticity can also be observed as changes in behaviour. In response to infection, both vertebrates and invertebrates practice self-medication, which can be considered a form of adaptive plasticity.[42] Various species of non-human primates infected with intestinal worms engage in leaf-swallowing, in which they ingest rough, whole leaves that physically dislodge parasites from the intestine. Additionally, the leaves irritate the gastric mucosa, which promotes the secretion of gastric acid and increases gut motility, effectively flushing parasites from the system.[43] The term "self-induced adaptive plasticity" has been used to describe situations in which a behavior under selection causes changes in subordinate traits that in turn enhance the ability of the organism to perform the behavior.[44] For example, birds that engage in altitudinal migration might make "trial runs" lasting a few hours that would induce physiological changes that would improve their ability to function at high altitude.[44]
Woolly bear caterpillars (Grammia incorrupta) infected with tachinid flies increase their survival by ingesting plants containing toxins known as pyrrolizidine alkaloids. The physiological basis for this change in behaviour is unknown; however, it is possible that, when activated, the immune system sends signals to the taste system that trigger plasticity in feeding responses during infection.[42]
Reproduction
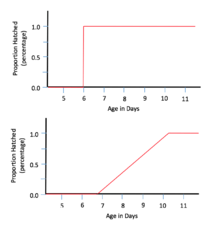
The red-eyed tree frog, Agalychnis callidryas, is an arboreal frog (hylid) that resides in the tropics of Central America. Unlike many frogs, the red-eyed tree frog has arboreal eggs which are laid on leaves hanging over ponds or large puddles and, upon hatching, the tadpoles fall into the water below. One of the most common predators encountered by these arboreal eggs is the cat-eyed snake, Leptodeira septentrionalis. In order to escape predation, the red-eyed tree frogs have developed a form of adaptive plasticity, which can also be considered phenotypic plasticity, when it comes to hatching age; the clutch is able to hatch prematurely and survive outside of the egg five days after oviposition when faced with an immediate threat of predation. The egg clutches take in important information from the vibrations felt around them and use it to determine whether or not they are at risk of predation. In the event of a snake attack, the clutch identifies the threat by the vibrations given off which, in turn, stimulates hatching almost instantaneously. In a controlled experiment conducted by Karen Warkentin, hatching rate and ages of red-eyed tree frogs were observed in clutches that were and were not attacked by the cat-eyed snake. When a clutch was attacked at six days of age, the entire clutch hatched at the same time, almost instantaneously. However, when a clutch is not presented with the threat of predation, the eggs hatch gradually over time with the first few hatching around seven days after oviposition, and the last of the clutch hatching around day ten. Karen Warkentin's study further explores the benefits and trade-offs of hatching plasticity in the red-eyed tree frog.[45]
Evolution
Plasticity is usually thought to be an evolutionary adaptation to environmental variation that is reasonably predictable and occurs within the lifespan of an individual organism, as it allows individuals to 'fit' their phenotype to different environments.[46][47] If the optimal phenotype in a given environment changes with environmental conditions, then the ability of individuals to express different traits should be advantageous and thus selected for. Hence, phenotypic plasticity can evolve if Darwinian fitness is increased by changing phenotype.[48][49] A similar logic should apply in artificial evolution attempting to introduce phenotypic plasticity to artificial agents.[50] However, the fitness benefits of plasticity can be limited by the energetic costs of plastic responses (e.g. synthesizing new proteins, adjusting expression ratio of isozyme variants, maintaining sensory machinery to detect changes) as well as the predictability and reliability of environmental cues[51] (see Beneficial acclimation hypothesis).
Freshwater snails (Physa virgata), provide an example of when phenotypic plasticity can be either adaptive or maladaptive. In the presence of a predator, bluegill sunfish, these snails make their shell shape more rotund and reduce growth. This makes them more crush-resistant and better protected from predation. However, these snails cannot tell the difference in chemical cues between the predatory and non-predatory sunfish. Thus, the snails respond inappropriately to non-predatory sunfish by producing an altered shell shape and reducing growth. These changes, in the absence of a predator, make the snails susceptible to other predators and limit fecundity. Therefore, these freshwater snails produce either an adaptive or maladaptive response to the environmental cue depending on whether the predatory sunfish is actually present.[52][53]
Given the profound ecological importance of temperature and its predictable variability over large spatial and temporal scales, adaptation to thermal variation has been hypothesized to be a key mechanism dictating the capacity of organisms for phenotypic plasticity.[54] The magnitude of thermal variation is thought to be directly proportional to plastic capacity, such that species that have evolved in the warm, constant climate of the tropics have a lower capacity for plasticity compared to those living in variable temperate habitats. Termed the "climatic variability hypothesis", this idea has been supported by several studies of plastic capacity across latitude in both plants and animals.[55][56] However, recent studies of Drosophila species have failed to detect a clear pattern of plasticity over latitudinal gradients, suggesting this hypothesis may not hold true across all taxa or for all traits.[57] Some researchers propose that direct measures of environmental variability, using factors such as precipitation, are better predictors of phenotypic plasticity than latitude alone.[58]
Selection experiments and experimental evolution approaches have shown that plasticity is a trait that can evolve when under direct selection and also as a correlated response to selection on the average values of particular traits.[59]
Plasticity and climate change
Unprecedented rates of climate change are predicted to occur over the next 100 years as a result of human activity.[60] Phenotypic plasticity is a key mechanism with which organisms can cope with a changing climate, as it allows individuals to respond to change within their lifetime.[61] This is thought to be particularly important for species with long generation times, as evolutionary responses via natural selection may not produce change fast enough to mitigate the effects of a warmer climate.
The North American red squirrel (Tamiasciurus hudsonicus) has experienced an increase in average temperature over this last decade of almost 2 °C. This increase in temperature has caused an increase in abundance of white spruce cones, the main food source for winter and spring reproduction. In response, the mean lifetime parturition date of this species has advanced by 18 days. Food abundance showed a significant effect on the breeding date with individual females, indicating a high amount of phenotypic plasticity in this trait.[62]
See also
- Acclimation
- Allometric engineering
- Baldwin effect
- Beneficial acclimation hypothesis
- Developmental biology
- Evolutionary physiology
- Genetic assimilation
- Rapoport's rule
References
- Price TD, Qvarnström A, Irwin DE (July 2003). "The role of phenotypic plasticity in driving genetic evolution". Proceedings: Biological Sciences. 270 (1523): 1433–40. doi:10.1098/rspb.2003.2372. PMC 1691402. PMID 12965006.
- Kelly SA, Panhuis TM, Stoehr AM (2012). "Phenotypic Plasticity: Molecular Mechanisms and Adaptive Significance". Comprehensive Physiology. 2. pp. 1417–39. doi:10.1002/cphy.c110008. ISBN 978-0-470-65071-4. PMID 23798305.
- Schlichting CD (1986). "The Evolution of Phenotypic Plasticity in Plants". Annual Review of Ecology and Systematics. 17: 667–93. doi:10.1146/annurev.es.17.110186.003315.
- International Aphid Genomics Consortium (February 2010). Eisen JA (ed.). "Genome sequence of the pea aphid Acyrthosiphon pisum". PLoS Biology. 8 (2): e1000313. doi:10.1371/journal.pbio.1000313. PMC 2826372. PMID 20186266.
- Silvertown, Jonathan (1989). "The paradox of seed size and adaptation". Trends in Ecology & Evolution. 4 (1): 24–26. doi:10.1016/0169-5347(89)90013-x. PMID 21227308.
- Sultan SE (December 2000). "Phenotypic plasticity for plant development, function and life history". Trends in Plant Science. 5 (12): 537–42. doi:10.1016/S1360-1385(00)01797-0. PMID 11120476.
- Rozendaal DM, Hurtado VH, Poorter L (2006). "Plasticity in leaf traits of 38 tropical tree species in response to light; relationships with light demand and adult stature". Functional Ecology. 20 (2): 207–16. doi:10.1111/j.1365-2435.2006.01105.x. JSTOR 3806552.
- Lambers H, Poorter H (1992). "Inherent Variation in Growth Rate Between Higher Plants: A Search for Physiological Causes and Ecological Consequences". Advances in Ecological Research Volume 23. Advances in Ecological Research. 23. pp. 187–261. doi:10.1016/S0065-2504(08)60148-8. ISBN 978-0-12-013923-1.
- Alemán F, Nieves-Cordones M, Martínez V, Rubio F (2009). "Differential regulation of the HAK5 genes encoding the high-affinity K+ transporters of Thellungiella halophila and Arabidopsis thaliana". Environmental and Experimental Botany. 65 (2–3): 263–9. doi:10.1016/j.envexpbot.2008.09.011.
- Tallman G, Zhu J, Mawson BT, Amodeo G, Nouhi Z, Levy K, Zeiger E (1997). "Induction of CAM in Mesembryanthemum crystallinum Abolishes the Stomatal Response to Blue Light and Light-Dependent Zeaxanthin Formation in Guard Cell Chloroplasts". Plant and Cell Physiology. 38 (3): 236–42. doi:10.1093/oxfordjournals.pcp.a029158.
- Li, Xin; Guo, Tingting; Mu, Qi; Li, Xianran; Yu, Jianming (2018-06-07). "Genomic and environmental determinants and their interplay underlying phenotypic plasticity". Proceedings of the National Academy of Sciences. 115 (26): 6679–6684. doi:10.1073/pnas.1718326115. ISSN 0027-8424. PMC 6042117. PMID 29891664.
- Chitwood, Daniel H.; Sinha, Neelima R. (April 2016). "Evolutionary and Environmental Forces Sculpting Leaf Development". Current Biology. 26 (7): R297–R306. doi:10.1016/j.cub.2016.02.033. no-break space character in
|first2=at position 8 (help); no-break space character in|first1=at position 7 (help) - Fritz, Michael André; Rosa, Stefanie; Sicard, Adrien (24 October 2018). "Mechanisms Underlying the Environmentally Induced Plasticity of Leaf Morphology". Frontiers in Genetics. 9: 478. doi:10.3389/fgene.2018.00478. PMC 6207588. PMID 30405690.
- Maugarny-Calès, Aude; Laufs, Patrick (10 July 2018). "Getting leaves into shape: a molecular, cellular, environmental and evolutionary view". Development. 145 (13): dev161646. doi:10.1242/dev.161646. PMID 29991476.
- Nakayama, Hokuto; Sinha, Neelima R.; Kimura, Seisuke (4 October 2017). "How Do Plants and Phytohormones Accomplish Heterophylly, Leaf Phenotypic Plasticity, in Response to Environmental Cues". Frontiers in Plant Science. 8: 1717. doi:10.3389/fpls.2017.01717. PMC 5632738. PMID 29046687.
- Kuwabara, A (2003). "Effects of ethylene and abscisic acid upon heterophylly in Ludwigia arcuata (Onagraceae)". Planta. 217 (6): 880–887. doi:10.1007/s00425-003-1062-z. PMID 12844266.
- Weaver ME, Ingram DL (1969). "Morphological Changes in Swine Associated with Environmental Temperature". Ecology. 50 (4): 710–3. doi:10.2307/1936264. JSTOR 1936264.
- Ostrander EA, Wayne RK (December 2005). "The canine genome". Genome Research. 15 (12): 1706–16. doi:10.1101/gr.3736605. PMID 16339369.
- Miklosi, Adam. Dog Behaviour, Evolution, and Cognition. 2007 Oxford University Press, Chapter 11.3
- Shreeve, T.G. (1987). "The mate location behaviour of the male speckled wood butterfly, Pararge aegeria, and the effect of phenotypic differences in hind-wing spotting". Animal Behaviour. 35 (3): 682–690. doi:10.1016/s0003-3472(87)80104-5.
- Guayasamin J, Krynak T, Krynak K, Culebras J, Hutter C (2015). "Phenotypic plasticity raises questions for taxonomically important traits: a remarkable new Andean rainfrog (Pristimantis) with the ability to change skin texture". Zoological Journal of the Linnean Society. 173 (4): 913–928. doi:10.1111/zoj.12222.
- Tremblay, Yann (2003). "Geographic variation in the foraging behaviour, diet and chick growth of rockhopper penguins" (PDF). Marine Ecology.
- Hazel JR (1995). "Thermal adaptation in biological membranes: is homeoviscous adaptation the explanation?". Annual Review of Physiology. 57: 19–42. doi:10.1146/annurev.ph.57.030195.000315. PMID 7778864.
- Brzek P, Kohl K, Caviedes-Vidal E, Karasov WH (May 2009). "Developmental adjustments of house sparrow (Passer domesticus) nestlings to diet composition". The Journal of Experimental Biology. 212 (Pt 9): 1284–93. doi:10.1242/jeb.023911. PMID 19376949.
- Cortés PA, Franco M, Sabat P, Quijano SA, Nespolo RF (October 2011). "Bioenergetics and intestinal phenotypic flexibility in the microbiotherid marsupial (Dromiciops gliroides) from the temperate forest in South America". Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology. 160 (2): 117–24. doi:10.1016/j.cbpa.2011.05.014. PMID 21627996.
- Starck JM (November 1999). "Phenotypic flexibility of the avian gizzard: rapid, reversible and repeated changes of organ size in response to changes in dietary fibre content". The Journal of Experimental Biology. 202 (22): 3171–9. PMID 10539966.
- Liu QS, Wang DH (July 2007). "Effects of diet quality on phenotypic flexibility of organ size and digestive function in Mongolian gerbils (Meriones unguiculatus)". Journal of Comparative Physiology B. 177 (5): 509–18. doi:10.1007/s00360-007-0149-4. PMID 17333208.
- Naya DE, Ebensperger LA, Sabat P, Bozinovic F (2008). "Digestive and metabolic flexibility allows female degus to cope with lactation costs". Physiological and Biochemical Zoology. 81 (2): 186–94. doi:10.1086/527453. PMID 18190284.
- Krockenberger AK, Hume ID (2007). "A flexible digestive strategy accommodates the nutritional demands of reproduction in a free-living folivore, the Koala (Phascolarctos cinereus)". Functional Ecology. 21 (4): 748–756. doi:10.1111/j.1365-2435.2007.01279.x.
- Hammond KA, Wunder BA (1991). "The Role of Diet Quality and Energy Need in the Nutritional Ecology of a Small Herbivore, Microtus ochrogaster". Physiological Zoology. 64 (2): 541–67. doi:10.1086/physzool.64.2.30158190. JSTOR 30158190.
- Sabat P, Riveros JM, López-Pinto C (January 2005). "Phenotypic flexibility in the intestinal enzymes of the African clawed frog Xenopus laevis". Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology. 140 (1): 135–9. doi:10.1016/j.cbpb.2004.11.010. PMID 15664322.
- Drobney RD (1984). "Effect of Diet on Visceral Morphology of Breeding Wood Ducks". The Auk. 101 (1): 93–8. doi:10.1093/auk/101.1.93. JSTOR 4086226.
- Kehoe FP, Ankney CD, Alisauskas RT (1988). "Effects of dietary fiber and diet diversity on digestive organs of captive Mallards (Anas platyrhynchos)". Canadian Journal of Zoology. 66 (7): 1597–602. doi:10.1139/z88-233.
- Starck JM, Beese K (January 2001). "Structural flexibility of the intestine of Burmese python in response to feeding". The Journal of Experimental Biology. 204 (Pt 2): 325–35. PMID 11136618.
- Freedman AH, Gronau I, Schweizer RM, Ortega-Del Vecchyo D, Han E, Silva PM, Galaverni M, Fan Z, Marx P, Lorente-Galdos B, Beale H, Ramirez O, Hormozdiari F, Alkan C, Vilà C, Squire K, Geffen E, Kusak J, Boyko AR, Parker HG, Lee C, Tadigotla V, Wilton A, Siepel A, Bustamante CD, Harkins TT, Nelson SF, Ostrander EA, Marques-Bonet T, Wayne RK, Novembre J (January 2014). "Genome sequencing highlights the dynamic early history of dogs". PLoS Genetics. 10 (1): e1004016. doi:10.1371/journal.pgen.1004016. PMC 3894170. PMID 24453982.
- Chadwick W, Little TJ (March 2005). "A parasite-mediated life-history shift in Daphnia magna". Proceedings: Biological Sciences. 272 (1562): 505–9. doi:10.1098/rspb.2004.2959. PMC 1578704. PMID 15799946.
- Ahmed AM, Baggott SL, Maingon R, Hurd H (2002). "The costs of mounting an immune response are reflected in the reproductive fitness of the mosquito Anopheles gambiae". Oikos. 97 (3): 371–377. doi:10.1034/j.1600-0706.2002.970307.x.
- Hurd H (August 2001). "Host fecundity reduction: a strategy for damage limitation?". Trends in Parasitology. 17 (8): 363–8. doi:10.1016/S1471-4922(01)01927-4. PMID 11685895.
- Schallig HD, Hordijk PL, Oosthoek PW, Jong-Brink M (1991). "Schistosomin, a peptide present in the haemolymph of Lymnaea stagnal is infected with Trichobilharzia ocellata, is produced only in the snail's central nervous system". Parasitology Research. 77 (2): 152–6. doi:10.1007/BF00935429.
- Forbes MR (1993). "Parasitism and Host Reproductive Effort". Oikos. 67 (3): 444–50. doi:10.2307/3545356. JSTOR 3545356.
- Kristan DM, Hammond KA (2003). "Physiological and morphological responses to simultaneous cold exposure and parasite infection by wild-derived house mice". Functional Ecology. 17 (4): 464–471. doi:10.1046/j.1365-2435.2003.00751.x. JSTOR 3598983.
- Singer MS, Mace KC, Bernays EA (2009). May RC (ed.). "Self-medication as adaptive plasticity: increased ingestion of plant toxins by parasitized caterpillars". PLOS ONE. 4 (3): e4796. doi:10.1371/journal.pone.0004796. PMC 2652102. PMID 19274098.
- Huffman MA (2001). "Self-Medicative Behavior in the African Great Apes: An Evolutionary Perspective into the Origins of Human Traditional Medicine". BioScience. 51 (8): 651–61. doi:10.1641/0006-3568(2001)051[0651:SMBITA]2.0.CO;2.
- Swallow JG, Rhodes JS, Garland T (June 2005). "Phenotypic and evolutionary plasticity of organ masses in response to voluntary exercise in house mice". Integrative and Comparative Biology. 45 (3): 426–37. doi:10.1093/icb/45.3.426. PMID 21676788.
- Warkentin KM (April 1995). "Adaptive plasticity in hatching age: a response to predation risk trade-offs". Proceedings of the National Academy of Sciences of the United States of America. 92 (8): 3507–10. doi:10.1073/pnas.92.8.3507. PMC 42196. PMID 11607529.
- Gabriel W (2005). "How stress selects for reversible phenotypic plasticity". Journal of Evolutionary Biology. 18 (4): 873–883. doi:10.1111/j.1420-9101.2005.00959.x. PMID 16033559.
- Garland T, Kelly SA (2006). "Phenotypic plasticity and experimental evolution". Journal of Experimental Biology. 209 (12): 2344–2361. doi:10.1242/jeb.02244. PMID 16731811.
- Gavrilets S, Scheiner S (1993). "The genetics of phenotypic plasticity. V. Evolution of reaction norm shape". Journal of Evolutionary Biology. 6: 31–48. doi:10.1046/j.1420-9101.1993.6010031.x.
- de Jong G (April 2005). "Evolution of phenotypic plasticity: patterns of plasticity and the emergence of ecotypes". The New Phytologist. 166 (1): 101–17. doi:10.1111/j.1469-8137.2005.01322.x. hdl:1874/10551. PMID 15760355.
- Hunt ER (2020). "Phenotypic Plasticity Provides a Bioinspiration Framework for Minimal Field Swarm Robotics". Frontiers in Robotics & AI. 7 (23). doi:10.3389/frobt.2020.00023.
- Dewitt TJ, Sih A, Wilson DS (1998). "Costs and limits of phenotypic plasticity". Trends in Ecology & Evolution. 13 (2): 77–81. doi:10.1016/s0169-5347(97)01274-3. PMID 21238209.
- Langerhans RB, DeWit TJ (2002). "Plasticity constrained: Over-generalized induction cues cause maladaptive phenotypes". Evolutionary Ecology Research. 4 (6): 857–70.
- Dewitt TJ, Sih A, Wilson DS (February 1998). "Costs and limits of phenotypic plasticity". Trends in Ecology & Evolution. 13 (2): 77–81. doi:10.1016/S0169-5347(97)01274-3. PMID 21238209.
- Janzen DH (1967). "Why Mountain Passes are Higher in the Tropics". The American Naturalist. 101 (919): 233–49. doi:10.1086/282487.
- Naya DE, Bozinovic F, Karasov WH (October 2008). "Latitudinal trends in digestive flexibility: testing the climatic variability hypothesis with data on the intestinal length of rodents". The American Naturalist. 172 (4): E122–34. doi:10.1086/590957. JSTOR 590957. PMID 18717635.
- Molina-Montenegro MA, Naya DE (2012). Seebacher F (ed.). "Latitudinal patterns in phenotypic plasticity and fitness-related traits: assessing the climatic variability hypothesis (CVH) with an invasive plant species". PLOS ONE. 7 (10): e47620. doi:10.1371/journal.pone.0047620. PMC 3478289. PMID 23110083.
- Overgaard J, Kristensen TN, Mitchell KA, Hoffmann AA (October 2011). "Thermal tolerance in widespread and tropical Drosophila species: does phenotypic plasticity increase with latitude?". The American Naturalist. 178 Suppl 1: S80–96. doi:10.1086/661780. PMID 21956094.
- Clements FE (1928). "The End of a World by Claude Anet". American Anthropologist. 30 (1): 125. doi:10.1525/aa.1928.30.1.02a00120. JSTOR 660970.
- Maldonado K, Bozinovic F, Rojas JM, Sabat P (2011). "Within-species digestive tract flexibility in rufous-collared sparrows and the climatic variability hypothesis". Physiological and Biochemical Zoology. 84 (4): 377–84. doi:10.1086/660970. PMID 21743251.
- IPCC, 2014: Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, R.K. Pachauri and L.A. Meyer (eds.)]. IPCC, Geneva, Switzerland, 151 pp. https://www.ipcc.ch/report/ar5/syr/
- Williams SE, Shoo LP, Isaac JL, Hoffmann AA, Langham G (December 2008). Moritz C (ed.). "Towards an integrated framework for assessing the vulnerability of species to climate change". PLoS Biology. 6 (12): 2621–6. doi:10.1371/journal.pbio.0060325. PMC 2605927. PMID 19108608.
- Réale D, McAdam AG, Boutin S, Berteaux D (March 2003). "Genetic and plastic responses of a northern mammal to climate change". Proceedings: Biological Sciences. 270 (1515): 591–6. doi:10.1098/rspb.2002.2224. JSTOR 3558706. PMC 1691280. PMID 12769458.
Further reading
- West-Eberhard MJ (2003). Developmental Plasticity and Evolution. Oxford University Press. ISBN 978-0-19-512234-3.
- Piersma T, Van Gils JA (2011). The Flexible Phenotype: A Body-Centred Integration of Ecology, Physiology, and Behaviour. Oxford University Press. ISBN 978-0-19-164015-5. See also: Garland T (2011). "The Flexible Phenotype: A Body-Centred Integration of Ecology, Physiology, and Behaviour". Animal Behaviour. 82 (3): 609–10. doi:10.1016/j.anbehav.2011.06.012.
External links
| Wikimedia Commons has media related to Phenotypic plasticity. |
- Special issue of the Journal of Experimental Biology concerning phenotypic plasticity
- Developmental Plasticity and Evolution - review of the book from American Scientist
- Isidro A. T. Savillo's Impermanence of Sexual Phenotypes from Biologybrowser (Thomson Reuters)
- Phenotypic Plasticity lecture from the Institute for the Development of Educational Applications
