Marine life
Marine life, or sea life or ocean life, is the plants, animals and other organisms that live in the salt water of the sea or ocean, or the brackish water of coastal estuaries. At a fundamental level, marine life affects the nature of the planet. Marine organisms produce oxygen and sequester carbon. Shorelines are in part shaped and protected by marine life, and some marine organisms even help create new land. The term marine comes from the Latin mare, meaning sea or ocean.

| Part of a series of overviews on |
| Marine life |
|---|
|
|
|
Most life forms evolved initially in marine habitats. By volume, oceans provide about 90 percent of the living space on the planet.[1] The earliest vertebrates appeared in the form of fish,[2] which live exclusively in water. Some of these evolved into amphibians which spend portions of their lives in water and portions on land. Other fish evolved into land mammals and subsequently returned to the ocean as seals, dolphins or whales. Plant forms such as kelp and algae grow in the water and are the basis for some underwater ecosystems. Plankton forms the general foundation of the ocean food chain, particularly the phytoplankton which are key primary producers.
Marine invertebrates exhibit a wide range of modifications to survive in poorly oxygenated waters, including breathing tubes as in mollusc siphons. Fish have gills instead of lungs, although some species of fish, such as the lungfish, have both. Marine mammals, such as dolphins, whales, otters, and seals need to surface periodically to breathe air.
There are over 200,000 documented marine species with perhaps two million marine species yet to be documented.[3] Marine species range in size from the microscopic, including phytoplankton which can be as small as 0.02 micrometres, to huge cetaceans (whales, dolphins and porpoises), including the blue whale – the largest known animal reaching 33 metres (108 ft) in length.[4][5] Marine microorganisms, including protists, bacteria and viruses, constitute about 70% of the total marine biomass.
Water
There is no life without water.[6] It has been described as the universal solvent for its ability to dissolve many substances,[7][8] and as the solvent of life.[9] Water is the only common substance to exist as a solid, liquid, and gas under conditions normal to life on Earth.[10] The Nobel Prize winner Albert Szent-Györgyi referred to water as the mater und matrix: the mother and womb of life.[11]
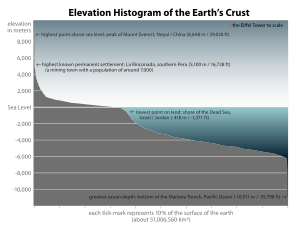
The abundance of surface water on Earth is a unique feature in the Solar System. Earth's hydrosphere consists chiefly of the oceans, but technically includes all water surfaces in the world, including inland seas, lakes, rivers, and underground waters down to a depth of 2,000 metres (6,600 ft) The deepest underwater location is Challenger Deep of the Mariana Trench in the Pacific Ocean, having a depth of 10,900 metres (6.8 mi).[note 1][12]
Conventionally the planet is divided into five separate oceans, but these oceans all connect into a single world ocean. The mass of this world ocean is 1.35×1018 metric tons, or about 1/4400 of Earth's total mass. The world ocean covers an area of 3.618×108 km2 with a mean depth of 3682 m, resulting in an estimated volume of 1.332×109 km3.[13] If all of Earth's crustal surface was at the same elevation as a smooth sphere, the depth of the resulting world ocean would be about 2.7 kilometres (1.7 mi).[14][15]
About 97.5% of the water on Earth is saline; the remaining 2.5% is fresh water. Most fresh water – about 69% – is present as ice in ice caps and glaciers.[16] The average salinity of Earth's oceans is about 35 grams (1.2 oz) of salt per kilogram of seawater (3.5% salt).[17] Most of the salt in the ocean comes from the weathering and erosion of rocks on land.[18] Some salts are released from volcanic activity or extracted from cool igneous rocks.[19]
The oceans are also a reservoir of dissolved atmospheric gases, which are essential for the survival of many aquatic life forms.[20] Sea water has an important influence on the world's climate, with the oceans acting as a large heat reservoir.[21] Shifts in the oceanic temperature distribution can cause significant weather shifts, such as the El Niño-Southern Oscillation.[22]

Altogether the ocean occupies 71 percent of the world surface,[1] averaging nearly 3.7 kilometres (2.3 mi) in depth.[23] By volume, the ocean provides about 90 percent of the living space on the planet.[1] The science fiction writer Arthur C. Clarke has pointed out it would be more appropriate to refer to planet Earth as planet Ocean.[24][25]
However water is found elsewhere in the solar system. Europa, one of the moons orbiting Jupiter, is slightly smaller than the Earth's moon. There is a strong possibility a large saltwater ocean exists beneath its ice surface.[26] It has been estimated the outer crust of solid ice is about 10–30 km (6–19 mi) thick and the liquid ocean underneath is about 100 km (60 mi) deep.[27] This would make Europa's ocean over twice the volume of the Earth's ocean. There has been speculation Europa's ocean could support life,[28][29] and could be capable of supporting multicellular microorganisms if hydrothermal vents are active on the ocean floor.[30] Enceladus, a small icy moon of Saturn, also has what appears to be an underground ocean which actively vents warm water from the moon's surface.[31]
Evolution
The Earth is about 4.54 billion years old.[32][33][34] The earliest undisputed evidence of life on Earth dates from at least 3.5 billion years ago,[35][36] during the Eoarchean Era after a geological crust started to solidify following the earlier molten Hadean Eon. Microbial mat fossils have been found in 3.48 billion-year-old sandstone in Western Australia.[37][38] Other early physical evidence of a biogenic substance is graphite in 3.7 billion-year-old metasedimentary rocks discovered in Western Greenland[39] as well as "remains of biotic life" found in 4.1 billion-year-old rocks in Western Australia.[40][41] According to one of the researchers, "If life arose relatively quickly on Earth … then it could be common in the universe."[40]
All organisms on Earth are descended from a common ancestor or ancestral gene pool.[42][43] Highly energetic chemistry is thought to have produced a self-replicating molecule around 4 billion years ago, and half a billion years later the last common ancestor of all life existed.[44] The current scientific consensus is that the complex biochemistry that makes up life came from simpler chemical reactions.[45] The beginning of life may have included self-replicating molecules such as RNA[46] and the assembly of simple cells.[47] In 2016 scientists reported a set of 355 genes from the last universal common ancestor (LUCA) of all life, including microorganisms, living on Earth.[48]
Current species are a stage in the process of evolution, with their diversity the product of a long series of speciation and extinction events.[49] The common descent of organisms was first deduced from four simple facts about organisms: First, they have geographic distributions that cannot be explained by local adaptation. Second, the diversity of life is not a set of completely unique organisms, but organisms that share morphological similarities. Third, vestigial traits with no clear purpose resemble functional ancestral traits and finally, that organisms can be classified using these similarities into a hierarchy of nested groups—similar to a family tree.[50] However, modern research has suggested that, due to horizontal gene transfer, this "tree of life" may be more complicated than a simple branching tree since some genes have spread independently between distantly related species.[51][52]
Past species have also left records of their evolutionary history. Fossils, along with the comparative anatomy of present-day organisms, constitute the morphological, or anatomical, record.[53] By comparing the anatomies of both modern and extinct species, paleontologists can infer the lineages of those species. However, this approach is most successful for organisms that had hard body parts, such as shells, bones or teeth. Further, as prokaryotes such as bacteria and archaea share a limited set of common morphologies, their fossils do not provide information on their ancestry.
- ^ Ciccarelli, Francesca D.; Doerks, Tobias; von Mering, Christian; et al. (3 March 2006). "Toward Automatic Reconstruction of a Highly Resolved Tree of Life". Science. 311 (5765): 1283–1287. Bibcode:2006Sci...311.1283C. CiteSeerX 10.1.1.381.9514. doi:10.1126/science.1123061. ISSN 0036-8075. PMID 16513982.
More recently, evidence for common descent has come from the study of biochemical similarities between organisms. For example, all living cells use the same basic set of nucleotides and amino acids.[54] The development of molecular genetics has revealed the record of evolution left in organisms' genomes: dating when species diverged through the molecular clock produced by mutations.[55] For example, these DNA sequence comparisons have revealed that humans and chimpanzees share 98% of their genomes and analysing the few areas where they differ helps shed light on when the common ancestor of these species existed.[56]
Prokaryotes inhabited the Earth from approximately 3–4 billion years ago.[57][58] No obvious changes in morphology or cellular organisation occurred in these organisms over the next few billion years.[59] The eukaryotic cells emerged between 1.6–2.7 billion years ago. The next major change in cell structure came when bacteria were engulfed by eukaryotic cells, in a cooperative association called endosymbiosis.[60][61] The engulfed bacteria and the host cell then underwent coevolution, with the bacteria evolving into either mitochondria or hydrogenosomes.[62] Another engulfment of cyanobacterial-like organisms led to the formation of chloroplasts in algae and plants.[63]
The history of life was that of the unicellular eukaryotes, prokaryotes and archaea until about 610 million years ago when multicellular organisms began to appear in the oceans in the Ediacaran period.[57][64] The evolution of multicellularity occurred in multiple independent events, in organisms as diverse as sponges, brown algae, cyanobacteria, slime moulds and myxobacteria.[65] In 2016 scientists reported that, about 800 million years ago, a minor genetic change in a single molecule called GK-PID may have allowed organisms to go from a single cell organism to one of many cells.[66]
Soon after the emergence of these first multicellular organisms, a remarkable amount of biological diversity appeared over a span of about 10 million years, in an event called the Cambrian explosion. Here, the majority of types of modern animals appeared in the fossil record, as well as unique lineages that subsequently became extinct.[67] Various triggers for the Cambrian explosion have been proposed, including the accumulation of oxygen in the atmosphere from photosynthesis.[68]
About 500 million years ago, plants and fungi started colonising the land. Evidence for the appearance of the first land plants occurs in the Ordovician, around 450 million years ago, in the form of fossil spores.[69] Land plants began to diversify in the Late Silurian, from around 430 million years ago.[70] The colonisation of the land by plants was soon followed by arthropods and other animals.[71] Insects were particularly successful and even today make up the majority of animal species.[72] Amphibians first appeared around 364 million years ago, followed by early amniotes and birds around 155 million years ago (both from "reptile"-like lineages), mammals around 129 million years ago, homininae around 10 million years ago and modern humans around 250,000 years ago.[73][74][75] However, despite the evolution of these large animals, smaller organisms similar to the types that evolved early in this process continue to be highly successful and dominate the Earth, with the majority of both biomass and species being prokaryotes.[76]
Estimates on the number of Earth's current species range from 10 million to 14 million,[77] of which about 1.2 million have been documented and over 86 percent have not yet been described.[78]
Microorganisms


Microorganisms make up about 70% of the marine biomass.[79] A microorganism, or microbe, is a microscopic organism too small to be recognised with the naked eye. It can be single-celled[80] or multicellular. Microorganisms are diverse and include all bacteria and archaea, most protozoa such as algae, fungi and certain microscopic animals such as rotifers.
Many macroscopic animals and plants have microscopic juvenile stages. Some microbiologists also classify viruses (and viroids) as microorganisms, but others consider these as nonliving.[81][82]
Microorganisms are crucial to nutrient recycling in ecosystems as they act as decomposers. Some microorganisms are pathogenic, causing disease and even death in plants and animals.[83] As inhabitants of the largest environment on Earth, microbial marine systems drive changes in every global system. Microbes are responsible for virtually all the photosynthesis that occurs in the ocean, as well as the cycling of carbon, nitrogen, phosphorus, other nutrients and trace elements.[84]
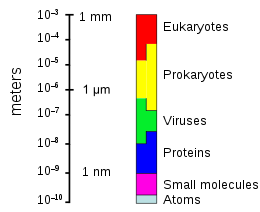
| Marine microorganisms |
| ||||||||||||||||||||||||
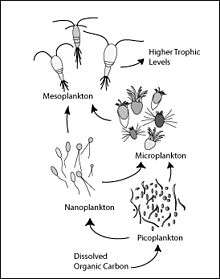
Microscopic life undersea is diverse and still poorly understood, such as for the role of viruses in marine ecosystems.[85] Most marine viruses are bacteriophages, which are harmless to plants and animals, but are essential to the regulation of saltwater and freshwater ecosystems.[86] They infect and destroy bacteria in aquatic microbial communities, and are the most important mechanism of recycling carbon in the marine environment. The organic molecules released from the dead bacterial cells stimulate fresh bacterial and algal growth.[87] Viral activity may also contribute to the biological pump, the process whereby carbon is sequestered in the deep ocean.[88]


A stream of airborne microorganisms circles the planet above weather systems but below commercial air lanes.[89] Some peripatetic microorganisms are swept up from terrestrial dust storms, but most originate from marine microorganisms in sea spray. In 2018, scientists reported that hundreds of millions of viruses and tens of millions of bacteria are deposited daily on every square meter around the planet.[90][91]
Microscopic organisms live throughout the biosphere. The mass of prokaryote microorganisms — which includes bacteria and archaea, but not the nucleated eukaryote microorganisms — may be as much as 0.8 trillion tons of carbon (of the total biosphere mass, estimated at between 1 and 4 trillion tons).[92] Single-celled barophilic marine microbes have been found at a depth of 10,900 m (35,800 ft) in the Mariana Trench, the deepest spot in the Earth's oceans.[93][94] Microorganisms live inside rocks 580 m (1,900 ft) below the sea floor under 2,590 m (8,500 ft) of ocean off the coast of the northwestern United States,[93][95] as well as 2,400 m (7,900 ft; 1.5 mi) beneath the seabed off Japan.[96] The greatest known temperature at which microbial life can exist is 122 °C (252 °F) (Methanopyrus kandleri).[97] In 2014, scientists confirmed the existence of microorganisms living 800 m (2,600 ft) below the ice of Antarctica.[98][99] According to one researcher, "You can find microbes everywhere — they're extremely adaptable to conditions, and survive wherever they are."[93]
Marine viruses
Viruses are small infectious agents that do not have their own metabolism and can replicate only inside the living cells of other organisms.[100] Viruses can infect all types of life forms, from animals and plants to microorganisms, including bacteria and archaea.[101] The linear size of the average virus is about one one-hundredth that of the average bacterium. Most viruses cannot be seen with an optical microscope so electron microscopes are used instead.[102]
Viruses are found wherever there is life and have probably existed since living cells first evolved.[103] The origin of viruses is unclear because they do not form fossils, so molecular techniques have been used to compare the DNA or RNA of viruses and are a useful means of investigating how they arise.[104]
Viruses are now recognised as ancient and as having origins that pre-date the divergence of life into the three domains.[105] But the origins of viruses in the evolutionary history of life are unclear: some may have evolved from plasmids—pieces of DNA that can move between cells—while others may have evolved from bacteria. In evolution, viruses are an important means of horizontal gene transfer, which increases genetic diversity.[106]
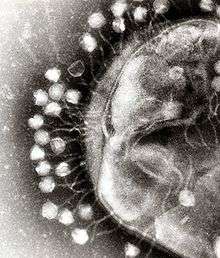
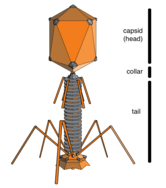
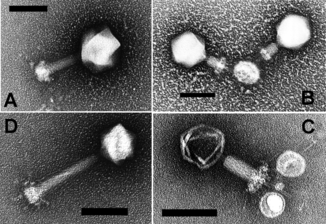
Opinions differ on whether viruses are a form of life or organic structures that interact with living organisms.[107] They are considered by some to be a life form, because they carry genetic material, reproduce by creating multiple copies of themselves through self-assembly, and evolve through natural selection. However they lack key characteristics such as a cellular structure generally considered necessary to count as life. Because they possess some but not all such qualities, viruses have been described as replicators[108] and as "organisms at the edge of life".[109]

Bacteriophages, often just called phages, are viruses that parasite bacteria and archaea. Marine phages parasite marine bacteria and archaea, such as cyanobacteria.[110] They are a common and diverse group of viruses and are the most abundant biological entity in marine environments, because their hosts, bacteria, are typically the numerically dominant cellular life in the sea. Generally there are about 1 million to 10 million viruses in each mL of seawater, or about ten times more double-stranded DNA viruses than there are cellular organisms,[111][112] although estimates of viral abundance in seawater can vary over a wide range.[113][114] Tailed bacteriophages appear to dominate marine ecosystems in number and diversity of organisms.[110] Bacteriophages belonging to the families Corticoviridae,[115] Inoviridae[116] and Microviridae[117] are also known to infect diverse marine bacteria.
Microorganisms make up about 70% of the marine biomass.[79] It is estimated viruses kill 20% of this biomass each day and that there are 15 times as many viruses in the oceans as there are bacteria and archaea. Viruses are the main agents responsible for the rapid destruction of harmful algal blooms,[118] which often kill other marine life.[119] The number of viruses in the oceans decreases further offshore and deeper into the water, where there are fewer host organisms.[88]
There are also archaean viruses which replicate within archaea: these are double-stranded DNA viruses with unusual and sometimes unique shapes.[120][121] These viruses have been studied in most detail in the thermophilic archaea, particularly the orders Sulfolobales and Thermoproteales.[122]
Viruses are an important natural means of transferring genes between different species, which increases genetic diversity and drives evolution.[106] It is thought that viruses played a central role in the early evolution, before the diversification of bacteria, archaea and eukaryotes, at the time of the last universal common ancestor of life on Earth.[123] Viruses are still one of the largest reservoirs of unexplored genetic diversity on Earth.[88]
Marine bacteria

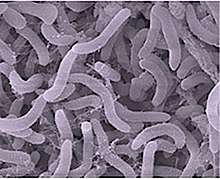
Bacteria constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a number of shapes, ranging from spheres to rods and spirals. Bacteria were among the first life forms to appear on Earth, and are present in most of its habitats. Bacteria inhabit soil, water, acidic hot springs, radioactive waste,[124] and the deep portions of Earth's crust. Bacteria also live in symbiotic and parasitic relationships with plants and animals.
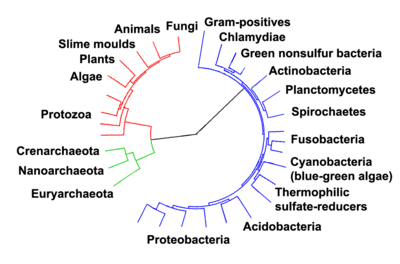
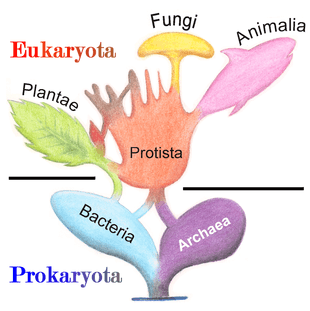
Once regarded as plants constituting the class Schizomycetes, bacteria are now classified as prokaryotes. Unlike cells of animals and other eukaryotes, bacterial cells do not contain a nucleus and rarely harbour membrane-bound organelles. Although the term bacteria traditionally included all prokaryotes, the scientific classification changed after the discovery in the 1990s that prokaryotes consist of two very different groups of organisms that evolved from an ancient common ancestor. These evolutionary domains are called Bacteria and Archaea.[125]
The ancestors of modern bacteria were unicellular microorganisms that were the first forms of life to appear on Earth, about 4 billion years ago. For about 3 billion years, most organisms were microscopic, and bacteria and archaea were the dominant forms of life.[126][127] Although bacterial fossils exist, such as stromatolites, their lack of distinctive morphology prevents them from being used to examine the history of bacterial evolution, or to date the time of origin of a particular bacterial species. However, gene sequences can be used to reconstruct the bacterial phylogeny, and these studies indicate that bacteria diverged first from the archaeal/eukaryotic lineage.[128] Bacteria were also involved in the second great evolutionary divergence, that of the archaea and eukaryotes. Here, eukaryotes resulted from the entering of ancient bacteria into endosymbiotic associations with the ancestors of eukaryotic cells, which were themselves possibly related to the Archaea.[61][129] This involved the engulfment by proto-eukaryotic cells of alphaproteobacterial symbionts to form either mitochondria or hydrogenosomes, which are still found in all known Eukarya. Later on, some eukaryotes that already contained mitochondria also engulfed cyanobacterial-like organisms. This led to the formation of chloroplasts in algae and plants. There are also some algae that originated from even later endosymbiotic events. Here, eukaryotes engulfed a eukaryotic algae that developed into a "second-generation" plastid.[130][131] This is known as secondary endosymbiosis.
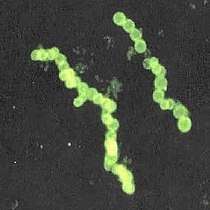 The marine Thiomargarita namibiensis, largest known bacterium
The marine Thiomargarita namibiensis, largest known bacterium Cyanobacteria blooms can contain lethal cyanotoxins
Cyanobacteria blooms can contain lethal cyanotoxins The chloroplasts of glaucophytes have a peptidoglycan layer, evidence suggesting their endosymbiotic origin from cyanobacteria.[132]
The chloroplasts of glaucophytes have a peptidoglycan layer, evidence suggesting their endosymbiotic origin from cyanobacteria.[132]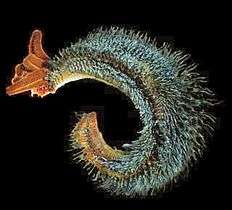 Bacteria can be beneficial. This Pompeii worm, an extremophile found only at hydrothermal vents, has a protective cover of bacteria.
Bacteria can be beneficial. This Pompeii worm, an extremophile found only at hydrothermal vents, has a protective cover of bacteria.
The largest known bacterium, the marine Thiomargarita namibiensis, can be visible to the naked eye and sometimes attains 0.75 mm (750 μm).[133][134]
Marine archaea

The archaea (Greek for ancient[136]) constitute a domain and kingdom of single-celled microorganisms. These microbes are prokaryotes, meaning they have no cell nucleus or any other membrane-bound organelles in their cells.
Archaea were initially classified as bacteria, but this classification is outdated.[137] Archaeal cells have unique properties separating them from the other two domains of life, Bacteria and Eukaryota. The Archaea are further divided into multiple recognized phyla. Classification is difficult because the majority have not been isolated in the laboratory and have only been detected by analysis of their nucleic acids in samples from their environment.
Archaea and bacteria are generally similar in size and shape, although a few archaea have very strange shapes, such as the flat and square-shaped cells of Haloquadratum walsbyi.[138] Despite this morphological similarity to bacteria, archaea possess genes and several metabolic pathways that are more closely related to those of eukaryotes, notably the enzymes involved in transcription and translation. Other aspects of archaeal biochemistry are unique, such as their reliance on ether lipids in their cell membranes, such as archaeols. Archaea use more energy sources than eukaryotes: these range from organic compounds, such as sugars, to ammonia, metal ions or even hydrogen gas. Salt-tolerant archaea (the Haloarchaea) use sunlight as an energy source, and other species of archaea fix carbon; however, unlike plants and cyanobacteria, no known species of archaea does both. Archaea reproduce asexually by binary fission, fragmentation, or budding; unlike bacteria and eukaryotes, no known species forms spores.
Archaea are particularly numerous in the oceans, and the archaea in plankton may be one of the most abundant groups of organisms on the planet. Archaea are a major part of Earth's life and may play roles in both the carbon cycle and the nitrogen cycle.
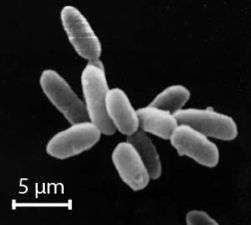 Halobacteria, found in water near saturated with salt, are now recognised as archaea.
Halobacteria, found in water near saturated with salt, are now recognised as archaea. Flat, square-shaped cells of the archaea Haloquadratum walsbyi
Flat, square-shaped cells of the archaea Haloquadratum walsbyi Methanosarcina barkeri, a marine archaea that produces methane
Methanosarcina barkeri, a marine archaea that produces methane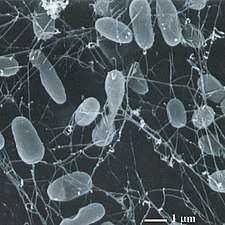 Thermophiles, such as Pyrolobus fumarii, survive well over 100 °C
Thermophiles, such as Pyrolobus fumarii, survive well over 100 °C Drawing of another marine thermophile, Pyrococcus furiosus
Drawing of another marine thermophile, Pyrococcus furiosus
Marine protists
Protists are eukaryotes that cannot be classified as plants, fungi or animals. They are usually single-celled and microscopic. Life originated as single-celled prokaryotes (bacteria and archaea) and later evolved into more complex eukaryotes. Eukaryotes are the more developed life forms known as plants, animals, fungi and protists. The term protist came into use historically as a term of convenience for eukaryotes that cannot be strictly classified as plants, animals or fungi. They are not a part of modern cladistics, because they are paraphyletic (lacking a common ancestor). Protists can be broadly divided into four groups depending on whether their nutrition is plant-like, animal-like, fungus-like,[139] or a mixture of these.[140]
Protists according to how they get food | |||||||
|---|---|---|---|---|---|---|---|
| Type of protist | Description | Example | Other examples | ||||
| Plant-like | Autotrophic protists that make their own food without needing to consume other organisms, usually by using photosynthesis | 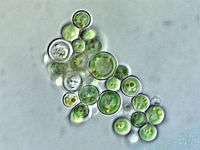 |
Red algae, Cyanidium sp. | Green algae, brown algae, diatoms and some dinoflagellates. Plant-like protists are important components of phytoplankton discussed below. | |||
| Animal-like | Protozoans |
Heterotrophic protists that get their food consuming other organisms |  |
Radiolarian protist as drawn by Haeckel | Foraminiferans, and some marine amoebae, ciliates and flagellates. | ||
| Fungus-like | Slime moulds and slime nets |
Saprotrophic protists that get their food from the remains of organisms that have broken down and decayed |  |
Marine slime nets form labyrinthine networks of tubes in which amoeba without pseudopods can travel | Marine lichen | ||
| Mixotropes | Various |
Mixotrophic and osmotrophic protists that get their food from a combination of the above | _(cropped).jpg) |
Euglena mutabilis, a photosynthetic flagellate | Many marine mixotropes are found among protists, including among ciliates, Rhizaria and dinoflagellates [141] | ||

Protists are highly diverse organisms currently organised into 18 phyla, but are not easy to classify.[143][144] Studies have shown high protist diversity exists in oceans, deep sea-vents and river sediments, suggesting a large number of eukaryotic microbial communities have yet to be discovered.[145][146] There has been little research on mixotrophic protists, but recent studies in marine environments found mixotrophic protests contribute a significant part of the protist biomass.[141]
- Single-celled and microscopic protists
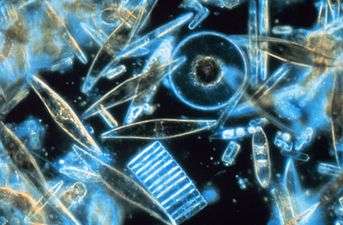

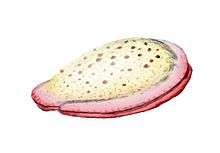
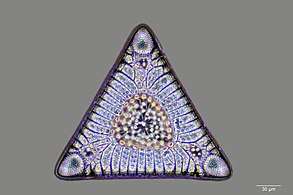 Fossil diatom frustule from 32-40 mya
Fossil diatom frustule from 32-40 mya.jpg) Radiolarian
Radiolarian.jpg) Single-celled alga, Gephyrocapsa oceanica
Single-celled alga, Gephyrocapsa oceanica Two dinoflagellates
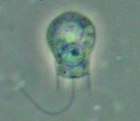
Two dinoflagellates Zooxanthellae is a photosynthetic algae that lives inside hosts like coral
Zooxanthellae is a photosynthetic algae that lives inside hosts like coral A single-celled ciliate with green zoochlorellae living inside endosymbiotically
A single-celled ciliate with green zoochlorellae living inside endosymbiotically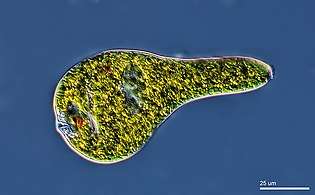 Euglenoid
Euglenoid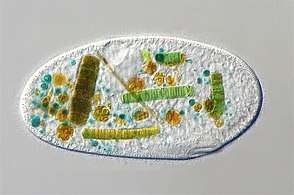 This ciliate is digesting cyanobacteria. The cytostome or mouth is at the bottom right.
This ciliate is digesting cyanobacteria. The cytostome or mouth is at the bottom right.
In contrast to the cells of prokaryotes, the cells of eukaryotes are highly organised. Plants, animals and fungi are usually multi-celled and are typically macroscopic. Most protists are single-celled and microscopic. But there are exceptions. Some single-celled marine protists are macroscopic. Some marine slime molds have unique life cycles that involve switching between unicellular, colonial, and multicellular forms.[149] Other marine protist are neither single-celled nor microscopic, such as seaweed.
- Macroscopic protists (see also unicellular macroalgae →)
 The single-celled giant amoeba has up to 1000 nuclei and reaches lengths of 5 mm
The single-celled giant amoeba has up to 1000 nuclei and reaches lengths of 5 mm Gromia sphaerica is a large spherical testate amoeba which makes mud trails. Its diameter is up to 3.8 cm.[150]
Gromia sphaerica is a large spherical testate amoeba which makes mud trails. Its diameter is up to 3.8 cm.[150]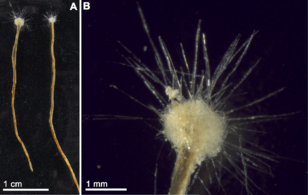 Spiculosiphon oceana, a unicellular foraminiferan with an appearance and lifestyle that mimics a sponge, grows to 5 cm long.
Spiculosiphon oceana, a unicellular foraminiferan with an appearance and lifestyle that mimics a sponge, grows to 5 cm long.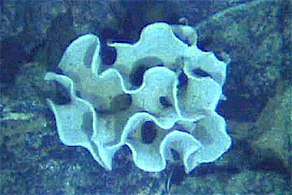 The xenophyophore, another single-celled foraminiferan, lives in abyssal zones. It has a giant shell up to 20 cm across.[151]
The xenophyophore, another single-celled foraminiferan, lives in abyssal zones. It has a giant shell up to 20 cm across.[151] Giant kelp, a brown algae, is not a true plant, yet it is multicellular and can grow to 50m
Giant kelp, a brown algae, is not a true plant, yet it is multicellular and can grow to 50m
Protists have been described as a taxonomic grab bag where anything that doesn't fit into one of the main biological kingdoms can be placed.[152] Some modern authors prefer to exclude multicellular organisms from the traditional definition of a protist, restricting protists to unicellular organisms.[153][154] This more constrained definition excludes seaweeds and slime molds.[155]
Marine microanimals
As juveniles, animals develop from microscopic stages, which can include spores, eggs and larvae. At least one microscopic animal group, the parasitic cnidarian Myxozoa, is unicellular in its adult form, and includes marine species. Other adult marine microanimals are multicellular. Microscopic adult arthropods are more commonly found inland in freshwater, but there are marine species as well. Microscopic adult marine crustaceans include some copepods, cladocera and tardigrades (water bears). Some marine nematodes and rotifers are also too small to be recognised with the naked eye, as are many loricifera, including the recently discovered anaerobic species that spend their lives in an anoxic environment.[156][157] Copepods contribute more to the secondary productivity and carbon sink of the world oceans than any other group of organisms.
- Marine microanimals
 Over 10,000 marine species are copepods, small, often microscopic crustaceans
Over 10,000 marine species are copepods, small, often microscopic crustaceans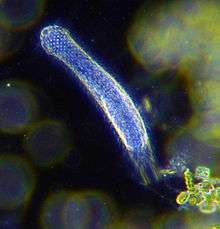 Darkfield photo of a gastrotrich, a worm-like animal living between sediment particles
Darkfield photo of a gastrotrich, a worm-like animal living between sediment particles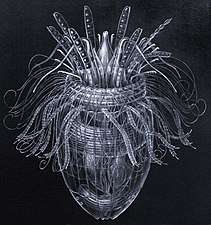 Armoured Pliciloricus enigmaticus, about 0.2 mm long, live in spaces between marine gravel
Armoured Pliciloricus enigmaticus, about 0.2 mm long, live in spaces between marine gravel Drawing of a tardigrade (water bear) on a grain of sand
Drawing of a tardigrade (water bear) on a grain of sand_-_160x_(13402418244).jpg) Rotifers, usually 0.1–0.5 mm long, may look like protists but have many cells and belongs to the Animalia
Rotifers, usually 0.1–0.5 mm long, may look like protists but have many cells and belongs to the Animalia
Fungi


Over 1500 species of fungi are known from marine environments.[158] These are parasitic on marine algae or animals, or are saprobes feeding on dead organic matter from algae, corals, protozoan cysts, sea grasses, wood and other substrata.[159] Spores of many species have special appendages which facilitate attachment to the substratum.[160] Marine fungi can also be found in sea foam and around hydrothermal areas of the ocean.[161] A diverse range of unusual secondary metabolites is produced by marine fungi.[162]
Mycoplankton are saprotropic members of the plankton communities of marine and freshwater ecosystems.[163][164] They are composed of filamentous free-living fungi and yeasts associated with planktonic particles or phytoplankton.[165] Similar to bacterioplankton, these aquatic fungi play a significant role in heterotrophic mineralization and nutrient cycling.[166] Mycoplankton can be up to 20 mm in diameter and over 50 mm in length.[167]
A typical milliliter of seawater contains about 103 to 104 fungal cells.[168] This number is greater in coastal ecosystems and estuaries due to nutritional runoff from terrestrial communities. A higher diversity of mycoplankton is found around coasts and in surface waters down to 1000 metres, with a vertical profile that depends on how abundant phytoplankton is.[169][170] This profile changes between seasons due to changes in nutrient availability.[171] Marine fungi survive in a constant oxygen deficient environment, and therefore depend on oxygen diffusion by turbulence and oxygen generated by photosynthetic organisms.[172]
Marine fungi can be classified as:[172]
- Lower fungi - adapted to marine habitats (zoosporic fungi, including mastigomycetes: oomycetes and chytridiomycetes)
- Higher fungi - filamentous, modified to planktonic lifestyle (hyphomycetes, ascomycetes, basidiomycetes). Most mycoplankton species are higher fungi.[169]
Lichens are mutualistic associations between a fungus, usually an ascomycete, and an alga or a cyanobacterium. Several lichens are found in marine environments.[173] Many more occur in the splash zone, where they occupy different vertical zones depending on how tolerant they are to submersion.[174] Some lichens live a long time; one species has been dated at 8,600 years.[175] However their lifespan is difficult to measure because what defines the same lichen is not precise.[176] Lichens grow by vegetatively breaking off a piece, which may or may not be defined as the same lichen, and two lichens of different ages can merge, raising the issue of whether it is the same lichen.[176]
The sea snail Littoraria irrorata damages plants of Spartina in the sea marshes where it lives, which enables spores of intertidal ascomycetous fungi to colonise the plant. The snail then eats the fungal growth in preference to the grass itself.[177]
According to fossil records, fungi date back to the late Proterozoic era 900-570 million years ago. Fossil marine lichens 600 million years old have been discovered in China.[178] It has been hypothesized that mycoplankton evolved from terrestrial fungi, likely in the Paleozoic era (390 million years ago).[179]
Origin of animals

The earliest animals were marine invertebrates, that is, vertebrates came later. Animals are multicellular eukaryotes,[note 2] and are distinguished from plants, algae, and fungi by lacking cell walls.[180] Marine invertebrates are animals that inhabit a marine environment apart from the vertebrate members of the chordate phylum; invertebrates lack a vertebral column. Some have evolved a shell or a hard exoskeleton.
The earliest animal fossils may belong to the genus Dickinsonia,[181] 571 million to 541 million years ago.[182] Individual Dickinsonia typically resemble a bilaterally symmetrical ribbed oval. They kept growing until they were covered with sediment or otherwise killed,[183] and spent most of their lives with their bodies firmly anchored to the sediment.[184] Their taxonomic affinities are presently unknown, but their mode of growth is consistent with a bilaterian affinity.[185]
Apart from Dickinsonia, the earliest widely accepted animal fossils are the rather modern-looking cnidarians (the group that includes coral, jellyfish, sea anemones and Hydra), possibly from around 580 Ma[186] The Ediacara biota, which flourished for the last 40 million years before the start of the Cambrian,[187] were the first animals more than a very few centimetres long. Like Dickinsonia, many were flat with a "quilted" appearance, and seemed so strange that there was a proposal to classify them as a separate kingdom, Vendozoa.[188] Others, however, have been interpreted as early molluscs (Kimberella[189][190]), echinoderms (Arkarua[191]), and arthropods (Spriggina,[192] Parvancorina[193]). There is still debate about the classification of these specimens, mainly because the diagnostic features which allow taxonomists to classify more recent organisms, such as similarities to living organisms, are generally absent in the Ediacarans. However, there seems little doubt that Kimberella was at least a triploblastic bilaterian animal, in other words, an animal significantly more complex than the cnidarians.[194]
Small shelly fauna are a very mixed collection of fossils found between the Late Ediacaran and Middle Cambrian periods. The earliest, Cloudina, shows signs of successful defense against predation and may indicate the start of an evolutionary arms race. Some tiny Early Cambrian shells almost certainly belonged to molluscs, while the owners of some "armor plates," Halkieria and Microdictyon, were eventually identified when more complete specimens were found in Cambrian lagerstätten that preserved soft-bodied animals.[195]
Body plans and phyla
Invertebrates are grouped into different phyla. Informally phyla can be thought of as a way of grouping organisms according to their body plan.[196][197]:33 A body plan refers to a blueprint which describes the shape or morphology of an organism, such as its symmetry, segmentation and the disposition of its appendages. The idea of body plans originated with vertebrates, which were grouped into one phylum. But the vertebrate body plan is only one of many, and invertebrates consist of many phyla or body plans. The history of the discovery of body plans can be seen as a movement from a worldview centred on vertebrates, to seeing the vertebrates as one body plan among many. Among the pioneering zoologists, Linnaeus identified two body plans outside the vertebrates; Cuvier identified three; and Haeckel had four, as well as the Protista with eight more, for a total of twelve. For comparison, the number of phyla recognised by modern zoologists has risen to 35.[197]

Historically body plans were thought of as having evolved rapidly during the Cambrian explosion,[201] but a more nuanced understanding of animal evolution suggests a gradual development of body plans throughout the early Palaeozoic and beyond.[202] More generally a phylum can be defined in two ways: as described above, as a group of organisms with a certain degree of morphological or developmental similarity (the phenetic definition), or a group of organisms with a certain degree of evolutionary relatedness (the phylogenetic definition).[202]
In the 1970s there was already a debate about whether the emergence of the modern phyla was "explosive" or gradual but hidden by the shortage of Precambrian animal fossils.[195] A re-analysis of fossils from the Burgess Shale lagerstätte increased interest in the issue when it revealed animals, such as Opabinia, which did not fit into any known phylum. At the time these were interpreted as evidence that the modern phyla had evolved very rapidly in the Cambrian explosion and that the Burgess Shale's "weird wonders" showed that the Early Cambrian was a uniquely experimental period of animal evolution.[203] Later discoveries of similar animals and the development of new theoretical approaches led to the conclusion that many of the "weird wonders" were evolutionary "aunts" or "cousins" of modern groups[204]—for example that Opabinia was a member of the lobopods, a group which includes the ancestors of the arthropods, and that it may have been closely related to the modern tardigrades.[205] Nevertheless, there is still much debate about whether the Cambrian explosion was really explosive and, if so, how and why it happened and why it appears unique in the history of animals.[206]
Basal invertebrate animals
The most basal animal phyla, the animals that evolved first, are the Porifera, Ctenophora, Placozoa and Cnidaria. None of these basal body plans exhibit bilateral symmetry.
| Animalia |
| |||||||||||||||||||||||||||
| 760 mya |
Marine sponges
.jpg)
Sponges are animals of the phylum Porifera (from Modern Latin for bearing pores[210]). They are multicellular organisms that have bodies full of pores and channels allowing water to circulate through them, consisting of jelly-like mesohyl sandwiched between two thin layers of cells. They have unspecialized cells that can transform into other types and that often migrate between the main cell layers and the mesohyl in the process. Sponges do not have nervous, digestive or circulatory systems. Instead, most rely on maintaining a constant water flow through their bodies to obtain food and oxygen and to remove wastes.
Sponges are similar to other animals in that they are multicellular, heterotrophic, lack cell walls and produce sperm cells. Unlike other animals, they lack true tissues and organs, and have no body symmetry. The shapes of their bodies are adapted for maximal efficiency of water flow through the central cavity, where it deposits nutrients, and leaves through a hole called the osculum. Many sponges have internal skeletons of spongin and/or spicules of calcium carbonate or silicon dioxide. All sponges are sessile aquatic animals. Although there are freshwater species, the great majority are marine (salt water) species, ranging from tidal zones to depths exceeding 8,800 m (5.5 mi). Some sponges live to great ages; there is evidence of the deep-sea glass sponge Monorhaphis chuni living about 11,000 years.[211][212]
While most of the approximately 5,000–10,000 known species feed on bacteria and other food particles in the water, some host photosynthesizing micro-organisms as endosymbionts and these alliances often produce more food and oxygen than they consume. A few species of sponge that live in food-poor environments have become carnivores that prey mainly on small crustaceans.[213]
 Sponge biodiversity. There are four sponge species in this photo.
Sponge biodiversity. There are four sponge species in this photo..jpg) Branching vase sponge
Branching vase sponge.jpg) Venus' flower basket at a depth of 2572 meters
Venus' flower basket at a depth of 2572 meters.jpg) Barrel sponge
Barrel sponge The long living Monorhaphis chuni
The long living Monorhaphis chuni
Linnaeus mistakenly identified sponges as plants in the order Algae.[214] For a long time thereafter sponges were assigned to a separate subkingdom, Parazoa (meaning beside the animals).[215] They are now classified as a paraphyletic phylum from which the higher animals have evolved.[216]
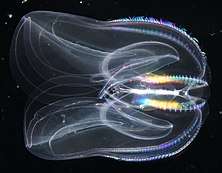
Ctenophores

Ctenophores (from Greek for carrying a comb), commonly known as comb jellies, are a phylum that live worldwide in marine waters. They are the largest non-colonial animals to swim with the help of cilia (hairs or combs).[217] Coastal species need to be tough enough to withstand waves and swirling sediment, but some oceanic species are so fragile and transparent that it is very difficult to capture them intact for study.[218] In the past ctenophores were thought to have only a modest presence in the ocean, but it is now known they are often significant and even dominant parts of the planktonic biomass.[219]:269
The phylum has about 150 known species with a wide range of body forms. Sizes range from a few millimeters to 1.5 m (4 ft 11 in). Cydippids are egg-shaped with their cilia arranged in eight radial comb rows, and deploy retractable tentacles for capturing prey. The benthic platyctenids are generally combless and flat. The coastal beroids have gaping mouths and lack tentacles. Most adult ctenophores prey on microscopic larvae and rotifers and small crustaceans but beroids prey on other ctenophores.
 Egg-shaped cydippid ctenophore
Egg-shaped cydippid ctenophore_on_Echniaster_luzonicus_(Seastar).jpg) Group of small benthic creeping comb jellies streaming tentacles and living symbiotically on a starfish
Group of small benthic creeping comb jellies streaming tentacles and living symbiotically on a starfish The beroid ctenophore, mouth gaping at left, preys on other ctenophores
The beroid ctenophore, mouth gaping at left, preys on other ctenophores The sea walnut has a transient anus which forms only when it needs to defecate[220]
The sea walnut has a transient anus which forms only when it needs to defecate[220]
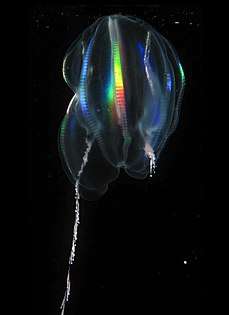 Light diffracting along the comb rows of a cydippid, left tentacle deployed, right retracted
Light diffracting along the comb rows of a cydippid, left tentacle deployed, right retracted Deep-sea ctenophore trailing tentacles studded with tentilla (sub-tentacles)
Deep-sea ctenophore trailing tentacles studded with tentilla (sub-tentacles)
Early writers combined ctenophores with cnidarians. Ctenophores resemble cnidarians in relying on water flow through the body cavity for both digestion and respiration, as well as in having a decentralized nerve net rather than a brain. Also like cnidarians, the bodies of ctenophores consist of a mass of jelly, with one layer of cells on the outside and another lining the internal cavity. In ctenophores, however, these layers are two cells deep, while those in cnidarians are only a single cell deep. While cnidarians exhibit radial symmetry, ctenophores have two anal canals which exhibit biradial symmetry (half-turn rotational symmetry).[221] The position of the ctenophores in the evolutionary family tree of animals has long been debated, and the majority view at present, based on molecular phylogenetics, is that cnidarians and bilaterians are more closely related to each other than either is to ctenophores.[219]:222
Placozoa
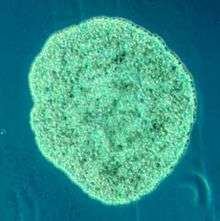
Placozoa (from Greek for flat animals) have the simplest structure of all animals. They are a basal form of free-living (non-parasitic) multicellular organism[222] that do not yet have a common name.[223] They form a phylum containing sofar only three described species, of which the first, the classical Trichoplax adhaerens, was discovered in 1883.[224] Two more species have been discovered since 2017,[225][226] and genetic methods indicate this phylum has a further 100 to 200 undescribed species.[227]
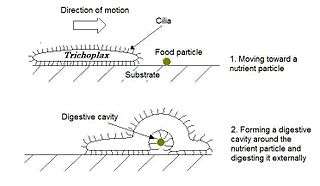
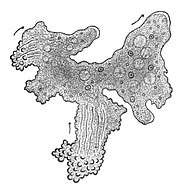
Trichoplax is a small, flattened, animal about one mm across and usually about 25 µm thick. Like the amoebae they superficially resemble, they continually change their external shape. In addition, spherical phases occasionally form which may facilitate movement. Trichoplax lacks tissues and organs. There is no manifest body symmetry, so it is not possible to distinguish anterior from posterior or left from right. It is made up of a few thousand cells of six types in three distinct layers.[228] The outer layer of simple epithelial cells bear cilia which the animal uses to help it creep along the seafloor.[229] Trichoplax feed by engulfing and absorbing food particles – mainly microbes and organic detritus – with their underside.
Marine cnidarians
_999_(30695685804).jpg)
Cnidarians (from Greek for nettle) are distinguished by the presence of stinging cells, specialized cells that they use mainly for capturing prey. Cnidarians include corals, sea anemones, jellyfish and hydrozoans. They form a phylum containing over 10,000[230] species of animals found exclusively in aquatic (mainly marine) environments. Their bodies consist of mesoglea, a non-living jelly-like substance, sandwiched between two layers of epithelium that are mostly one cell thick. They have two basic body forms: swimming medusae and sessile polyps, both of which are radially symmetrical with mouths surrounded by tentacles that bear cnidocytes. Both forms have a single orifice and body cavity that are used for digestion and respiration.
Fossil cnidarians have been found in rocks formed about 580 million years ago. Fossils of cnidarians that do not build mineralized structures are rare. Scientists currently think cnidarians, ctenophores and bilaterians are more closely related to calcareous sponges than these are to other sponges, and that anthozoans are the evolutionary "aunts" or "sisters" of other cnidarians, and the most closely related to bilaterians.
Cnidarians are the simplest animals in which the cells are organised into tissues.[231] The starlet sea anemone is used as a model organism in research.[232] It is easy to care for in the laboratory and a protocol has been developed which can yield large numbers of embryos on a daily basis.[233] There is a remarkable degree of similarity in the gene sequence conservation and complexity between the sea anemone and vertebrates.[233] In particular, genes concerned in the formation of the head in vertebrates are also present in the anemone.[234][235]
 Sea anemones are common in tidepools
Sea anemones are common in tidepools Their tentacles sting and paralyse small fish
Their tentacles sting and paralyse small fish Close up of polyps on the surface of a coral, waving their tentacles.
Close up of polyps on the surface of a coral, waving their tentacles. If an island sinks below the sea, coral growth can keep up with rising water and form an atoll
If an island sinks below the sea, coral growth can keep up with rising water and form an atoll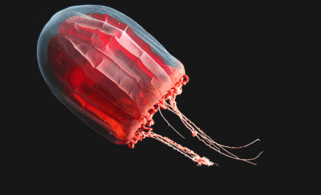 The mantle of the red paper lantern jellyfish crumples and expands like a paper lantern[236]
The mantle of the red paper lantern jellyfish crumples and expands like a paper lantern[236]
.jpg) The Portuguese man o' war is a colonial siphonophore
The Portuguese man o' war is a colonial siphonophore Marrus orthocanna another colonial siphonophore, assembled from two types of zooids
Marrus orthocanna another colonial siphonophore, assembled from two types of zooids Porpita porpita consists of a colony of hydroids[237]
Porpita porpita consists of a colony of hydroids[237] Lion's mane jellyfish, largest known jellyfish[238]
Lion's mane jellyfish, largest known jellyfish[238].jpg) Turritopsis dohrnii achieves biological immortality by transferring its cells back to childhood [239][240]
Turritopsis dohrnii achieves biological immortality by transferring its cells back to childhood [239][240].jpg) The sea wasp is the most lethal jellyfish in the world[241]
The sea wasp is the most lethal jellyfish in the world[241]
Bilateral invertebrate animals
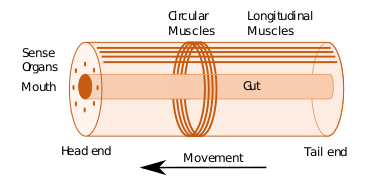
Some of the earliest bilaterians were wormlike, and the original bilaterian may have been a bottom dwelling worm with a single body opening.[242] A bilaterian body can be conceptualized as a cylinder with a gut running between two openings, the mouth and the anus. Around the gut it has an internal body cavity, a coelom or pseudocoelom.[lower-alpha 1] Animals with this bilaterally symmetric body plan have a head (anterior) end and a tail (posterior) end as well as a back (dorsal) and a belly (ventral); therefore they also have a left side and a right side.[243][244]
Having a front end means that this part of the body encounters stimuli, such as food, favouring cephalisation, the development of a head with sense organs and a mouth.[245] The body stretches back from the head, and many bilaterians have a combination of circular muscles that constrict the body, making it longer, and an opposing set of longitudinal muscles, that shorten the body;[244] these enable soft-bodied animals with a hydrostatic skeleton to move by peristalsis.[246] They also have a gut that extends through the basically cylindrical body from mouth to anus. Many bilaterian phyla have primary larvae which swim with cilia and have an apical organ containing sensory cells. However, there are exceptions to each of these characteristics; for example, adult echinoderms are radially symmetric (unlike their larvae), and certain parasitic worms have extremely simplified body structures.[243][244]
.jpg)
| ← bilaterians |
| |||||||||||||||||||||
Protostomes
Protostomes (from Greek for first mouth) are a superphylum of animals. It is a sister clade of the deuterostomes (from Greek for second mouth), with which it forms the Nephrozoa clade. Protostomes are distinguished from deuterostomes by the way their embryos develop. In protostomes the first opening that develops becomes the mouth, while in deuterostomes it becomes the anus.[249][250]
| ← Protostomes |
| ||||||||||||||||||||||||||||||||||||||||||
| (extant) |
Marine worms

Worms (Old English for serpents) form a number of phyla. Different groups of marine worms are related only distantly, so they are found in several different phyla such as the Annelida (segmented worms), Chaetognatha (arrow worms), Phoronida (horseshoe worms), and Hemichordata. All worms, apart from the Hemichordata, are protostomes. The Hemichordata are deuterostomes and are discussed in their own section below.
The typical body plan of a worm involves long cylindrical tube-like bodies and no limbs. Marine worms vary in size from microscopic to over 1 metre (3.3 ft) in length for some marine polychaete worms (bristle worms)[251] and up to 58 metres (190 ft) for the marine nemertean worm (bootlace worm).[252] Some marine worms occupy a small variety of parasitic niches, living inside the bodies of other animals, while others live more freely in the marine environment or by burrowing underground. Many of these worms have specialized tentacles used for exchanging oxygen and carbon dioxide and also may be used for reproduction. Some marine worms are tube worms, such as the giant tube worm which lives in waters near underwater volcanoes and can withstand temperatures up to 90 degrees Celsius. Platyhelminthes (flatworms) form another worm phylum which includes a class of parasitic tapeworms. The marine tapeworm Polygonoporus giganticus, found in the gut of sperm whales, can grow to over 30 m (100 ft).[253][254]
Nematodes (roundworms) constitute a further worm phylum with tubular digestive systems and an opening at both ends.[255][256] Over 25,000 nematode species have been described,[257][258] of which more than half are parasitic. It has been estimated another million remain undescribed.[259] They are ubiquitous in marine, freshwater and terrestrial environments, where they often outnumber other animals in both individual and species counts. They are found in every part of the earth's lithosphere, from the top of mountains to the bottom of oceanic trenches.[260] By count they represent 90% of all animals on the ocean floor.[261] Their numerical dominance, often exceeding a million individuals per square meter and accounting for about 80% of all individual animals on earth, their diversity of life cycles, and their presence at various trophic levels point at an important role in many ecosystems.[262]
 Giant tube worms cluster around hydrothermal vents
Giant tube worms cluster around hydrothermal vents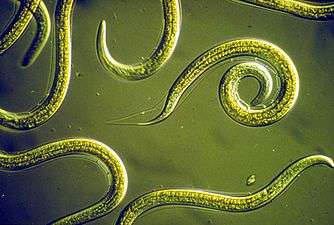 Nematodes are ubiquitous pseudocoelomates which can parasite marine plants and animals.
Nematodes are ubiquitous pseudocoelomates which can parasite marine plants and animals. Bloodworms are typically found on the bottom of shallow marine waters
Bloodworms are typically found on the bottom of shallow marine waters
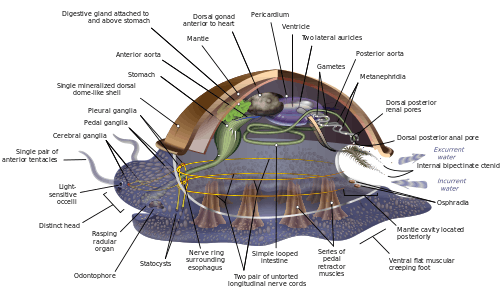
Marine molluscs



Molluscs (Latin for soft) form a phylum with about 85,000 extant recognized species.[265] They are the largest marine phylum in terms of species count, containing about 23% of all the named marine organisms.[266] Molluscs have more varied forms than other invertebrate phyla. They are highly diverse, not just in size and in anatomical structure, but also in behaviour and in habitat.
The mollusc phylum is divided into 9 or 10 taxonomic classes. These classes include gastropods, bivalves and cephalopods, as well as other lesser-known but distinctive classes. Gastropods with protective shells are referred to as snails, whereas gastropods without protective shells are referred to as slugs. Gastropods are by far the most numerous molluscs in terms of species.[267] Bivalves include clams, oysters, cockles, mussels, scallops, and numerous other families. There are about 8,000 marine bivalves species (including brackish water and estuarine species). A deep sea ocean quahog clam has been reported as having lived 507 years[268] making it the longest recorded life of all animals apart from colonial animals, or near-colonial animals like sponges.[211]
- Gastropods and bivalves
 Marine gastropods are sea snails or sea slugs. This nudibranch is a sea slug.
Marine gastropods are sea snails or sea slugs. This nudibranch is a sea slug.- The sea snail Syrinx aruanus has a shell up to 91 cm long, the largest of any living gastropod

 Common mussel, another bivalve
Common mussel, another bivalve
Cephalopods include octopus, squid and cuttlefish. About 800 living species of marine cephalopods have been identified,[269] and an estimated 11,000 extinct taxa have been described.[270] They are found in all oceans, but there are no fully freshwater cephalopods.[271]
- Cephalopods
- The nautilus is a living fossil little changed since it evolved 500 million years ago as one of the first cephalopods.[272][273][274]
 Reconstruction of an ammonite, a highly successful early cephalopod that appeared 400 mya
Reconstruction of an ammonite, a highly successful early cephalopod that appeared 400 mya- Cephalopods, like this cuttlefish, use their mantle cavity for jet propulsion
 Colossal squid, largest of all invertebrates[275]
Colossal squid, largest of all invertebrates[275]
Molluscs have such diverse shapes that many textbooks base their descriptions of molluscan anatomy on a generalized or hypothetical ancestral mollusc. This generalized mollusc is unsegmented and bilaterally symmetrical with an underside consisting of a single muscular foot. Beyond that it has three further key features. Firstly, it has a muscular cloak called a mantle covering its viscera and containing a significant cavity used for breathing and excretion. A shell secreted by the mantle covers the upper surface. Secondly (apart from bivalves) it has a rasping tongue called a radula used for feeding. Thirdly, it has a nervous system including a complex digestive system using microscopic, muscle-powered hairs called cilia to exude mucus. The generalized mollusc has two paired nerve cords (three in bivalves). The brain, in species that have one, encircles the esophagus. Most molluscs have eyes and all have sensors detecting chemicals, vibrations, and touch.[276][277]
Good evidence exists for the appearance of marine gastropods, cephalopods and bivalves in the Cambrian period 541 to 485.4 million years ago.
Marine arthropods
Arthropods (Greek for jointed feet) have an exoskeleton (external skeleton), a segmented body, and jointed appendages (paired appendages). They form a phylum which includes insects, arachnids, myriapods, and crustaceans. Arthropods are characterized by their jointed limbs and cuticle made of chitin, often mineralised with calcium carbonate. The arthropod body plan consists of segments, each with a pair of appendages. The rigid cuticle inhibits growth, so arthropods replace it periodically by moulting. Their versatility has enabled them to become the most species-rich members of all ecological guilds in most environments.
The evolutionary ancestry of arthropods dates back to the Cambrian period and is generally regarded as monophyletic. However, basal relationships of arthropods with extinct phyla such as lobopodians have recently been debated.[281][282]
| Panarthropoda |
| ||||||||||||
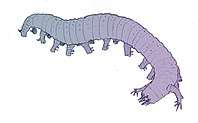

- Arthropod fossils and living fossils
 Fossil trilobite. Trilobites first appeared about 521 Ma. They were highly successful and were found everywhere in the ocean for 270 Ma.[284]
Fossil trilobite. Trilobites first appeared about 521 Ma. They were highly successful and were found everywhere in the ocean for 270 Ma.[284]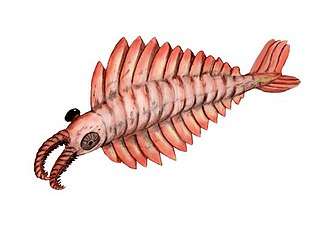 The Anomalocaris ("abnormal shrimp") was one of the first apex predators and first appeared about 515 Ma
The Anomalocaris ("abnormal shrimp") was one of the first apex predators and first appeared about 515 Ma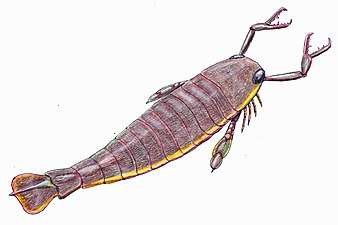 The largest known arthropod, the sea scorpion Jaekelopterus rhenaniae, has been found in estuarine strata from about 390 Ma. It was up to 2.5 m (8.2 ft) long.[285][286]
The largest known arthropod, the sea scorpion Jaekelopterus rhenaniae, has been found in estuarine strata from about 390 Ma. It was up to 2.5 m (8.2 ft) long.[285][286].jpg) Horseshoe crabs are living fossils, essentially unchanged for 450 Ma
Horseshoe crabs are living fossils, essentially unchanged for 450 Ma
Extant marine arthropods range in size from the microscopic crustacean Stygotantulus to the Japanese spider crab. Arthropods' primary internal cavity is a hemocoel, which accommodates their internal organs, and through which their haemolymph - analogue of blood - circulates; they have open circulatory systems. Like their exteriors, the internal organs of arthropods are generally built of repeated segments. Their nervous system is "ladder-like", with paired ventral nerve cords running through all segments and forming paired ganglia in each segment. Their heads are formed by fusion of varying numbers of segments, and their brains are formed by fusion of the ganglia of these segments and encircle the esophagus. The respiratory and excretory systems of arthropods vary, depending as much on their environment as on the subphylum to which they belong.
- Modern crustaceans
 Many crustaceans are very small, like this tiny amphipod, and make up a significant part of the ocean's zooplankton
Many crustaceans are very small, like this tiny amphipod, and make up a significant part of the ocean's zooplankton The Japanese spider crab has the longest leg span of any arthropod, reaching 5.5 metres (18 ft) from claw to claw.[287]
The Japanese spider crab has the longest leg span of any arthropod, reaching 5.5 metres (18 ft) from claw to claw.[287]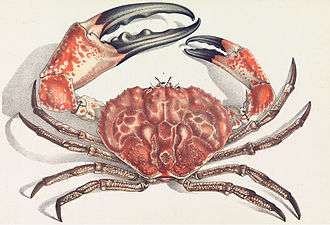 The Tasmanian giant crab is long-lived and slow-growing, making it vulnerable to overfishing.[288]
The Tasmanian giant crab is long-lived and slow-growing, making it vulnerable to overfishing.[288] Mantis shrimp have the most advanced eyes in the animal kingdom,[289] and smash prey by swinging their club-like raptorial claws.[290]
Mantis shrimp have the most advanced eyes in the animal kingdom,[289] and smash prey by swinging their club-like raptorial claws.[290]
Arthropod vision relies on various combinations of compound eyes and pigment-pit ocelli: in most species the ocelli can only detect the direction from which light is coming, and the compound eyes are the main source of information. Arthropods also have a wide range of chemical and mechanical sensors, mostly based on modifications of the many setae (bristles) that project through their cuticles. Arthropod methods of reproduction are diverse: terrestrial species use some form of internal fertilization while marine species lay eggs using either internal or external fertilization. Arthropod hatchlings vary from miniature adults to grubs that lack jointed limbs and eventually undergo a total metamorphosis to produce the adult form.
Deuterostomes
In deuterostomes the first opening that develops in the growing embryo becomes the anus, while in protostomes it becomes the mouth. Deuterostomes form a superphylum of animals and are the sister clade of the protostomes.[249][250] The earliest known deuterostomes are Saccorhytus fossils from about 540 million years ago. The Saccorhytus mouth may have functioned also as its anus.[291]
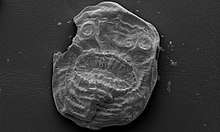
| ← deuterostomes |
| ||||||||||||||||||||||||
| (extant) |
Echinoderms
Echinoderms (Greek for spiny skin) is a phylum which contains only marine invertebrates. The phylum contains about 7000 living species,[292] making it the second-largest grouping of deuterostomes, after the chordates.
Adult echinoderms are recognizable by their radial symmetry (usually five-point) and include starfish, sea urchins, sand dollars, and sea cucumbers, as well as the sea lilies.[293] Echinoderms are found at every ocean depth, from the intertidal zone to the abyssal zone. They are unique among animals in having bilateral symmetry at the larval stage, but fivefold symmetry (pentamerism, a special type of radial symmetry) as adults.[294]
Echinoderms are important both biologically and geologically. Biologically, there are few other groupings so abundant in the biotic desert of the deep sea, as well as shallower oceans. Most echinoderms are able to regenerate tissue, organs, limbs, and reproduce asexually; in some cases, they can undergo complete regeneration from a single limb. Geologically, the value of echinoderms is in their ossified skeletons, which are major contributors to many limestone formations, and can provide valuable clues as to the geological environment. They were the most used species in regenerative research in the 19th and 20th centuries.
 Echinoderm literally means "spiny skin", as this water melon sea urchin illustrates
Echinoderm literally means "spiny skin", as this water melon sea urchin illustrates
- Colorful sea lilies in shallow waters
 Sea cucumbers filter feed on plankton and suspended solids
Sea cucumbers filter feed on plankton and suspended solids.jpg) The sea pig, a deep water sea cucumber, is the only echinoderm that uses legged locomotion
The sea pig, a deep water sea cucumber, is the only echinoderm that uses legged locomotion The benthopelagic sea cucumber can lift off the seafloor and journey as much as 1,000 m (3,300 ft) up the water column
The benthopelagic sea cucumber can lift off the seafloor and journey as much as 1,000 m (3,300 ft) up the water column
It is held by some scientists that the radiation of echinoderms was responsible for the Mesozoic Marine Revolution. Aside from the hard-to-classify Arkarua (a Precambrian animal with echinoderm-like pentamerous radial symmetry), the first definitive members of the phylum appeared near the start of the Cambrian.
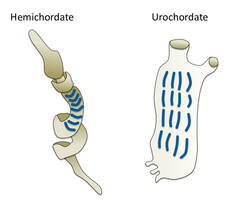
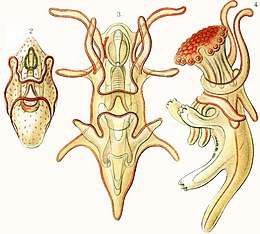
Hemichordates
Hemichordates form a sister phylum to the echinoderms. They are solitary worm-shaped organisms rarely seen by humans because of their lifestyle. They include two main groups, the acorn worms and the Pterobranchia. Pterobranchia form a class containing about 30 species of small worm-shaped animals that live in secreted tubes on the ocean floor. Acorn worms form a class containing about 111 species that generally live in U-shaped burrows on the seabed, from the shoreline to a depth of 3000 metres. The worms lie there with the proboscis sticking out of one opening in the burrow, subsisting as deposit feeders or suspension feeders. It is supposed the ancestors of acorn worms used to live in tubes like their relatives, the Pterobranchia, but eventually started to live a safer and more sheltered existence in sediment burrows.[298] Some of these worms may grow to be very long; one particular species may reach a length of 2.5 metres (8 ft 2 in), although most acorn worms are much smaller.
Acorn worms are more highly specialised and advanced than other worm-like organisms. They have a circulatory system with a heart that also functions as a kidney. Acorn worms have gill-like structures they use for breathing, similar to the gills of fish. Therefore, acorn worms are sometimes said to be a link between classical invertebrates and vertebrates. Acorn worms continually form new gill slits as they grow in size, and some older individuals have more than a hundred on each side. Each slit consists of a branchial chamber opening to the pharynx through a U-shaped cleft. Cilia push water through the slits, maintaining a constant flow, just as in fish.[299] Some acorn worms also have a postanal tail which may be homologous to the post-anal tail of vertebrates.
The three-section body plan of the acorn worm is no longer present in the vertebrates, except in the anatomy of the frontal neural tube, later developed into a brain divided into three parts. This means some of the original anatomy of the early chordate ancestors is still present in vertebrates even if it is not always visible. One theory is the three-part body originated from an early common ancestor of the deuterostomes, and maybe even from a common bilateral ancestor of both deuterostomes and protostomes. Studies have shown the gene expression in the embryo share three of the same signaling centers that shape the brains of all vertebrates, but instead of taking part in the formation of their neural system,[300] they are controlling the development of the different body regions.[301]
Marine chordates
The chordate phylum has three subphyla, one of which is the vertebrates (see below). The other two subphyla are marine invertebrates: the tunicates (salps and sea squirts) and the cephalochordates (such as lancelets). Invertebrate chordates are close relatives to vertebrates. In particular, there has been discussion about how closely some extinct marine species, such as Pikaiidae, Palaeospondylus, Zhongxiniscus and Vetulicolia, might relate ancestrally to vertebrates.
- Invertebrate chordates are close relatives of vertebrates
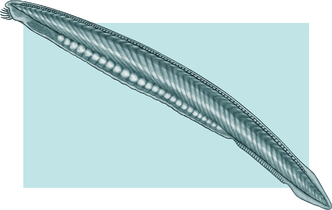
_(4_cm).png) Tunicates, like these fluorescent-colored sea squirts, may provide clues to vertebrate and therefore human ancestry.[304]
Tunicates, like these fluorescent-colored sea squirts, may provide clues to vertebrate and therefore human ancestry.[304]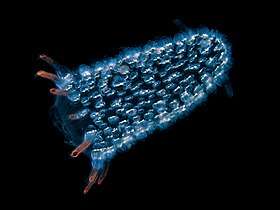 Pyrosomes are free-floating bioluminescent tunicates made up of hundreds of individuals
Pyrosomes are free-floating bioluminescent tunicates made up of hundreds of individuals.jpg) Salp chain
Salp chain
Vertebrate animals
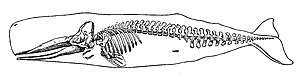
Vertebrates (Latin for joints of the spine) are a subphylum of chordates. They are chordates that have a vertebral column (backbone). The vertebral column provides the central support structure for an internal skeleton which gives shape, support, and protection to the body and can provide a means of anchoring fins or limbs to the body. The vertebral column also serves to house and protect the spinal cord that lies within the vertebral column.
Marine vertebrates can be divided into marine fish and marine tetrapods.
Marine fish
Fish typically breathe by extracting oxygen from water through gills and have a skin protected by scales and mucous. They use fins to propel and stabilise themselves in the water, and usually have a two-chambered heart and eyes well adapted to seeing underwater, as well as other sensory systems. Over 33,000 species of fish have been described as of 2017,[305] of which about 20,000 are marine fish.[306]
| ← vertebrates |
| |||||||||||||||||||||||||||
| (extant) |
Jawless fish
Early fish had no jaws. Most went extinct when they were outcompeted by jawed fish (below), but two groups survived: hagfish and lampreys. Hagfish form a class of about 20 species of eel-shaped, slime-producing marine fish. They are the only known living animals that have a skull but no vertebral column. Lampreys form a superclass containing 38 known extant species of jawless fish.[307] The adult lamprey is characterized by a toothed, funnel-like sucking mouth. Although they are well known for boring into the flesh of other fish to suck their blood,[308] only 18 species of lampreys are actually parasitic.[309] Together hagfish and lampreys are the sister group to vertebrates. Living hagfish remain similar to hagfish from around 300 million years ago.[310] The lampreys are a very ancient lineage of vertebrates, though their exact relationship to hagfishes and jawed vertebrates is still a matter of dispute.[311] Molecular analysis since 1992 has suggested that hagfish are most closely related to lampreys,[312] and so also are vertebrates in a monophyletic sense. Others consider them a sister group of vertebrates in the common taxon of craniata.[313]

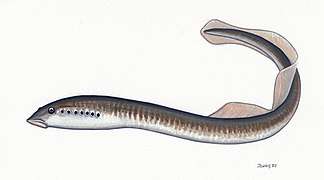 Lampreys are often parasitic and have a toothed, funnel-like sucking mouth
Lampreys are often parasitic and have a toothed, funnel-like sucking mouth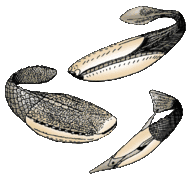 The extinct Pteraspidomorphi, ancestral to jawed vertebrates
The extinct Pteraspidomorphi, ancestral to jawed vertebrates
Pteraspidomorphi is an extinct class of early jawless fish ancestral to jawed vertebrates. The few characteristics they share with the latter are now considered as primitive for all vertebrates.
Around the start of the Devonian, fish started appearing with a deep remodelling of the vertebrate skull that resulted in a jaw.[314] All vertebrate jaws, including the human jaw, have evolved from these early fish jaws. The appearance of the early vertebrate jaw has been described as "perhaps the most profound and radical evolutionary step in vertebrate history".[315][316] Jaws make it possible to capture, hold, and chew prey. Fish without jaws had more difficulty surviving than fish with jaws, and most jawless fish became extinct during the Triassic period.
Cartilaginous fish
Jawed fish fall into two main groups: fish with bony internal skeletons and fish with cartilaginous internal skeletons. Cartilaginous fish, such as sharks and rays, have jaws and skeletons made of cartilage rather than bone. Megalodon is an extinct species of shark that lived about 28 to 1.5 Ma. It looked much like a stocky version of the great white shark, but was much larger with fossil lengths reaching 20.3 metres (67 ft).[317] Found in all oceans[318] it was one of the largest and most powerful predators in vertebrate history,[317] and probably had a profound impact on marine life.[319] The Greenland shark has the longest known lifespan of all vertebrates, about 400 years.[320] Some sharks such as the great white are partially warm blooded and give live birth. The manta ray, largest ray in the world, has been targeted by fisheries and is now vulnerable.[321]
- Cartilaginous fishes
 Cartilaginous fishes may have evolved from spiny sharks
Cartilaginous fishes may have evolved from spiny sharks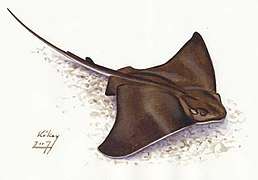
 The manta ray is the largest of all rays
The manta ray is the largest of all rays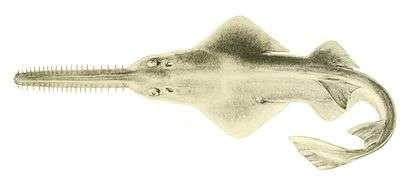 Sawfish are rays with long rostrums resembling a saw. All are now endangered or critically endangered[322]
Sawfish are rays with long rostrums resembling a saw. All are now endangered or critically endangered[322]
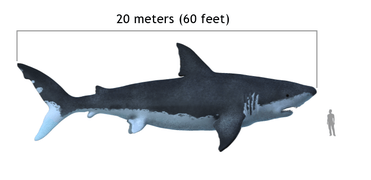 The extinct megalodon resembled a giant great white shark
The extinct megalodon resembled a giant great white shark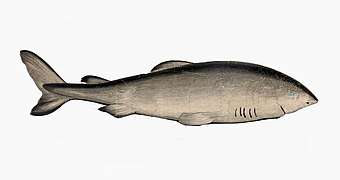 The Greenland shark lives longer than any other vertebrate
The Greenland shark lives longer than any other vertebrate.jpg)
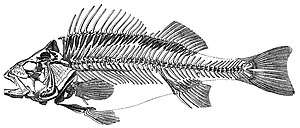
Bony fish

.jpg)
Bony fish have jaws and skeletons made of bone rather than cartilage. Bony fish also have hard, bony plates called operculum which help them respire and protect their gills, and they often possess a swim bladder which they use for better control of their buoyancy. Bony fish can be further divided into those with lobe fins and those with ray fins. The approximate dates in the phylogenetic tree are from Near et al., 2012[323] and Zhu et al., 2009.[324]
| ← bony fish |
| ||||||||||||||||||||||||||||||
| (extant) |
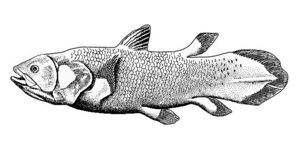
Lobe fins have the form of fleshy lobes supported by bony stalks which extend from the body.[325] Guiyu oneiros, the earliest-known bony fish, lived during the Late Silurian 419 million years ago. It has the combination of both ray-finned and lobe-finned features, although analysis of the totality of its features place it closer to lobe-finned fish.[324] Lobe fins evolved into the legs of the first tetrapod land vertebrates, so by extension an early ancestor of humans was a lobe-finned fish. Apart from the coelacanths and the lungfishes, lobe-finned fishes are now extinct.
The remaining bony fish have ray fins. These are made of webs of skin supported by bony or horny spines (rays) which can be erected to control the fin stiffness.
- The main distinguishing feature of the chondrosteans (sturgeon, paddlefish, bichir and reedfish) is the cartilaginous nature of their skeletons. The ancestors of the chondrosteans are thought to be bony fish, but the characteristic of an ossified skeleton was lost in later evolutionary development, resulting in a lightening of the frame.[326]
- Neopterygians (from Greek for new fins) appeared sometime in the Late Permian, before dinosaurs. They were a very successful group of fish, because they could move more rapidly than their ancestors. Their scales and skeletons began to lighten during their evolution, and their jaws became more powerful and efficient.[327]
Teleosts

About 96% of the world's fish species are teleosts,[328] of which about 14,000 are marine species.[329] Teleosts can be distinguished from other bony fish by their possession of a homocercal tail, a tail where the upper half mirrors the lower half.[330] Another difference lies in their jaw bones – teleosts have modifications in the jaw musculature which make it possible for them to protrude their jaws. This enables them to grab prey and draw it into their mouth.[330] In general, teleosts tend to be quicker and more flexible than more basal bony fishes. Their skeletal structure has evolved towards greater lightness. While teleost bones are well calcified, they are constructed from a scaffolding of struts, rather than the dense cancellous bones of holostean fish.[331]
Teleosts are found in almost all marine habitats.[332] They have enormous diversity, and range in size from adult gobies 8mm long [333] to ocean sunfish weighting over 2,000 kg.[334] The following images show something of the diversity in the shape and colour of modern marine teleosts...
 Sailfish
Sailfish Mahi-mahi
Mahi-mahi


 Pufferfish
Pufferfish Clown triggerfish
Clown triggerfish Mandarin dragonet
Mandarin dragonet
Marine tetrapods

A tetrapod (Greek for four feet) is a vertebrate with limbs (feet). Tetrapods evolved from ancient lobe-finned fishes about 400 million years ago during the Devonian Period when their earliest ancestors emerged from the sea and adapted to living on land.[335] This change from a body plan for breathing and navigating in gravity-neutral water to a body plan with mechanisms enabling the animal to breath in air without dehydrating and move on land is one of the most profound evolutionary changes known.[336][337] Tetrapods can be divided into four classes: amphibians, reptiles, birds and mammals.
| ← tetrapods |
| ||||||||||||||||||
Marine tetrapods are tetrapods that returned from land back to the sea again. The first returns to the ocean may have occurred as early as the Carboniferous Period[338] whereas other returns occurred as recently as the Cenozoic, as in cetaceans, pinnipeds,[339] and several modern amphibians.[340] Amphibians (from Greek for both kinds of life) live part of their life in water and part on land. They mostly require fresh water to reproduce. A few inhabit brackish water, but there are no true marine amphibians.[341] There have been reports, however, of amphibians invading marine waters, such as a Black Sea invasion by the natural hybrid Pelophylax esculentus reported in 2010.[342]
Reptiles
Reptiles (Late Latin for creeping or crawling) do not have an aquatic larval stage, and in this way are unlike amphibians. Most reptiles are oviparous, although several species of squamates are viviparous, as were some extinct aquatic clades[343] — the fetus develops within the mother, contained in a placenta rather than an eggshell. As amniotes, reptile eggs are surrounded by membranes for protection and transport, which adapt them to reproduction on dry land. Many of the viviparous species feed their fetuses through various forms of placenta analogous to those of mammals, with some providing initial care for their hatchlings.
Some reptiles are more closely related to birds than other reptiles, and many scientists prefer to make Reptilia a monophyletic group which includes the birds.[344][345][346][347] Extant non-avian reptiles which inhabit or frequent the sea include sea turtles, sea snakes, terrapins, the marine iguana, and the saltwater crocodile. Currently, of the approximately 12,000 extant reptile species and sub-species, only about 100 of are classed as marine reptiles.[348]
Except for some sea snakes, most extant marine reptiles are oviparous and need to return to land to lay their eggs. Apart from sea turtles, the species usually spend most of their lives on or near land rather than in the ocean. Sea snakes generally prefer shallow waters nearby land, around islands, especially waters that are somewhat sheltered, as well as near estuaries.[349][350] Unlike land snakes, sea snakes have evolved flattened tails which help them swim.[351]
 Marine iguana
Marine iguana.jpg)
.jpg)
 Marine snakes have flattened tails
Marine snakes have flattened tails The ancient Ichthyosaurus communis independently evolved flippers similar to dolphins
The ancient Ichthyosaurus communis independently evolved flippers similar to dolphins
Some extinct marine reptiles, such as ichthyosaurs, evolved to be viviparous and had no requirement to return to land. Ichthyosaurs resembled dolphins. They first appeared about 245 million years ago and disappeared about 90 million years ago. The terrestrial ancestor of the ichthyosaur had no features already on its back or tail that might have helped along the evolutionary process. Yet the ichthyosaur developed a dorsal and tail fin which improved its ability to swim.[352] The biologist Stephen Jay Gould said the ichthyosaur was his favourite example of convergent evolution.[353] The earliest marine reptiles arose in the Permian. During the Mesozoic many groups of reptiles became adapted to life in the seas, including ichthyosaurs, plesiosaurs, mosasaurs, nothosaurs, placodonts, sea turtles, thalattosaurs and thalattosuchians. Marine reptiles were less numerous after mass extinction at the end of the Cretaceous.
Birds
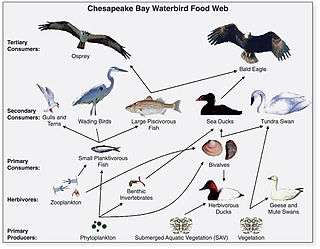
Marine birds are adapted to life within the marine environment. They are often called seabirds. While marine birds vary greatly in lifestyle, behaviour and physiology, they often exhibit striking convergent evolution, as the same environmental problems and feeding niches have resulted in similar adaptations. Examples include albatross, penguins, gannets, and auks.
In general, marine birds live longer, breed later and have fewer young than terrestrial birds do, but they invest a great deal of time in their young. Most species nest in colonies, which can vary in size from a few dozen birds to millions. Many species are famous for undertaking long annual migrations, crossing the equator or circumnavigating the Earth in some cases. They feed both at the ocean's surface and below it, and even feed on each other. Marine birds can be highly pelagic, coastal, or in some cases spend a part of the year away from the sea entirely. Some marine birds plummet from heights, plunging through the water leaving vapour-like trails, similar to that of fighter planes.[354] Gannets plunge into the water at up to 100 kilometres per hour (60 mph). They have air sacs under their skin in their face and chest which act like bubble-wrap, cushioning the impact with the water.
 European herring gull attack herring schools from above
European herring gull attack herring schools from above Gentoo penguin swimming underwater
Gentoo penguin swimming underwater Gannets "divebomb" at high speed
Gannets "divebomb" at high speed Albatrosses range over huge areas of ocean and regularly circle the globe.
Albatrosses range over huge areas of ocean and regularly circle the globe.
The first marine birds evolved in the Cretaceous period, and modern marine bird families emerged in the Paleogene.
Mammals

Mammals (from Latin for breast) are characterised by the presence of mammary glands which in females produce milk for feeding (nursing) their young. There are about 130 living and recently extinct marine mammal species such as seals, dolphins, whales, manatees, sea otters and polar bears.[355] They do not represent a distinct taxon or systematic grouping, but are instead unified by their reliance on the marine environment for feeding. Both cetaceans and sirenians are fully aquatic and therefore are obligate water dwellers. Seals and sea-lions are semiaquatic; they spend the majority of their time in the water, but need to return to land for important activities such as mating, breeding and molting. In contrast, both otters and the polar bear are much less adapted to aquatic living. Their diet varies considerably as well: some may eat zooplankton; others may eat fish, squid, shellfish, and sea-grass; and a few may eat other mammals.
In a process of convergent evolution, marine mammals, especially cetaceans redeveloped their body plan to parallel the streamlined fusiform body plan of pelagic fish. Front legs became flippers and back legs disappeared, a dorsal fin reappeared and the tail morphed into a powerful horizontal fluke. This body plan is an adaptation to being an active predator in a high drag environment. A parallel convergence occurred with the now extinct marine reptile ichthyosaur.[356]
 Endangered blue whale, largest animal ever[357]
Endangered blue whale, largest animal ever[357] Bottlenose dolphin, highest encephalization of any animal after humans[358]
Bottlenose dolphin, highest encephalization of any animal after humans[358]
 Dugong grazing on seagrass
Dugong grazing on seagrass
.jpg)
Primary producers
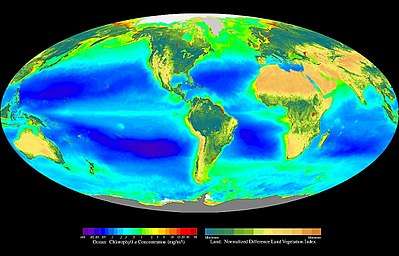
Primary producers are the autotroph organisms that make their own food instead of eating other organisms. This means primary producers become the starting point in the food chain for heterotroph organisms that do eat other organisms. Some marine primary producers are specialised bacteria and archaea which are chemotrophs, making their own food by gathering around hydrothermal vents and cold seeps and using chemosynthesis. However most marine primary production comes from organisms which use photosynthesis on the carbon dioxide dissolved in the water. This process uses energy from sunlight to convert water and carbon dioxide[359]:186–187 into sugars that can be used both as a source of chemical energy and of organic molecules that are used in the structural components of cells.[359]:1242 Marine primary producers are important because they underpin almost all marine animal life by generating most of the oxygen and food that provide other organisms with the chemical energy they need to exist.
The principal marine primary producers are cyanobacteria, algae and marine plants. The oxygen released as a by-product of photosynthesis is needed by nearly all living things to carry out cellular respiration. In addition, primary producers are influential in the global carbon and water cycles. They stabilize coastal areas and can provide habitats for marine animals. The term division has been traditionally used instead of phylum when discussing primary producers, but the International Code of Nomenclature for algae, fungi, and plants now accepts both terms as equivalents.[360]
Cyanobacteria
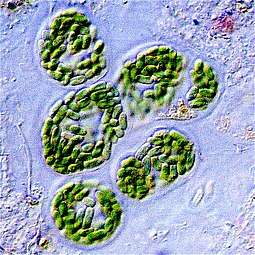
.jpg)
Cyanobacteria were the first organisms to evolve an ability to turn sunlight into chemical energy. They form a phylum (division) of bacteria which range from unicellular to filamentous and include colonial species. They are found almost everywhere on earth: in damp soil, in both freshwater and marine environments, and even on Antarctic rocks.[361] In particular, some species occur as drifting cells floating in the ocean, and as such were amongst the first of the phytoplankton.
The first primary producers that used photosynthesis were oceanic cyanobacteria about 2.3 billion years ago.[362][363] The release of molecular oxygen by cyanobacteria as a by-product of photosynthesis induced global changes in the Earth's environment. Because oxygen was toxic to most life on Earth at the time, this led to the near-extinction of oxygen-intolerant organisms, a dramatic change which redirected the evolution of the major animal and plant species.[364]
The tiny marine cyanobacterium Prochlorococcus, discovered in 1986, forms today part of the base of the ocean food chain and accounts for much of the photosynthesis of the open ocean[365] and an estimated 20% of the oxygen in the Earth's atmosphere.[366] It is possibly the most plentiful genus on Earth: a single millilitre of surface seawater may contain 100,000 cells or more.[367]
Originally, biologists classified cyanobacteria as algae, and referred to it as "blue-green algae". The more recent view is that cyanobacteria is a bacteria, and hence is not even in the same Kingdom as algae. Most authorities today exclude all prokaryotes, and hence cyanobacteria from the definition of algae.[368][369]
Algae
.jpg)
.jpg)


Algae is an informal term for a widespread and diverse group of photosynthetic protists which are not necessarily closely related and are thus polyphyletic. Marine algae can be divided into six groups:
- green algae, an informal group containing about 8,000 recognised species.[370] Many species live most of their lives as single cells or are filamentous, while others form colonies made up from long chains of cells, or are highly differentiated macroscopic seaweeds.
- red algae, a (disputed) phylum containing about 7,000 recognised species,[371] mostly multicellular and including many notable seaweeds.[371][372]
- brown algae, a class containing about 2,000 recognised species,[373] mostly multicellular and including many seaweeds, including kelp
- diatoms, a (disputed) phylum containing about 100,000 recognised species of mainly unicellular algae. Diatoms generate about 20 percent of the oxygen produced on the planet each year,[147] take in over 6.7 billion metric tons of silicon each year from the waters in which they live,[374] and contribute nearly half of the organic material found in the oceans. The shells (frustules) of dead diatoms can reach as much as half a mile deep on the ocean floor.[375]
- dinoflagellates, a phylum of unicellular flagellates with about 2,000 marine species.[376] Many dinoflagellates are known to be photosynthetic, but a large fraction of these are in fact mixotrophic, combining photosynthesis with ingestion of prey (phagotrophy).[377] Some species are endosymbionts of marine animals and play an important part in the biology of coral reefs. Others predate other protozoa, and a few forms are parasitic.
- euglenophytes, a phylum of unicellular flagellates with only a few marine members
Unlike higher plants, algae lack roots, stems, or leaves. They can be classified by size as microalgae or macroalgae.
Microalgae are the microscopic types of algae, not visible to the naked eye. They are mostly unicellular species which exist as individuals or in chains or groups, though some are multicellular. Microalgae are important components of the marine protists (discussed above), as well as the phytoplankton (discussed below). They are very diverse. It has been estimated there are 200,000-800,000 species of which about 50,000 species have been described.[378] Depending on the species, their sizes range from a few micrometers (µm) to a few hundred micrometers. They are specially adapted to an environment dominated by viscous forces.
.jpg) Chlamydomonas globosa, a unicellular green alga with two flagella just visible at bottom left
Chlamydomonas globosa, a unicellular green alga with two flagella just visible at bottom left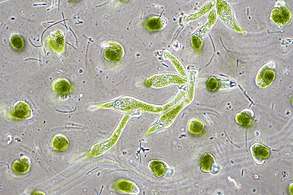 Chlorella vulgaris, a common green microalgae, in endosymbiosis with a ciliate [379]
Chlorella vulgaris, a common green microalgae, in endosymbiosis with a ciliate [379]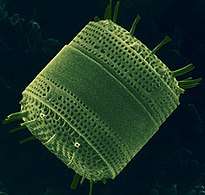 Centric diatom
Centric diatom Dinoflagellates
Dinoflagellates
Macroalgae are the larger, multicellular and more visible types of algae, commonly called seaweeds. Seaweeds usually grow in shallow coastal waters where they are anchored to the seafloor by a holdfast. Seaweed that becomes adrift can wash up on beaches. Kelp is a large brown seaweed that forms large underwater forests covering about 25% of the world coastlines.[380] They are among the most productive and dynamic ecosystems on Earth.[381] Some Sargassum seaweeds are planktonic (free-floating). Like microalgae, macroalgae (seaweeds) are technically marine protists since they are not true plants.
 A seaweed is a macroscopic form of
A seaweed is a macroscopic form of
red or brown or green algae- Sargassum seaweed is a planktonic brown alga with air bladders that help it float
 Sargassum fish are camouflaged to live among drifting Sargassum seaweed
Sargassum fish are camouflaged to live among drifting Sargassum seaweed

- Unicellular macroalgae (see also macroscopic protists ←)
 The unicellular mermaid's wineglass are mushroom-shaped algae that grow up to 10 cm high.
The unicellular mermaid's wineglass are mushroom-shaped algae that grow up to 10 cm high. Killer algae are single-celled organisms, but look like ferns and grow stalks up to 80 cm long.[383]
Killer algae are single-celled organisms, but look like ferns and grow stalks up to 80 cm long.[383]
Unicellular organisms are usually microscopic, less than one tenth of a millimeter long. There are exceptions. Mermaid's wineglass, a genus of subtropical green algae, is single-celled but remarkably large and complex in form with a single large nucleus, making it a model organism for studying cell biology.[384] Another single celled algae, Caulerpa taxifolia, has the appearance of a vascular plant including "leaves" arranged neatly up stalks like a fern. Selective breeding in aquariums to produce hardier strains resulted in an accidental release into the Mediterranean where it has become an invasive species known colloquially as killer algae.[385]
Origin of plants
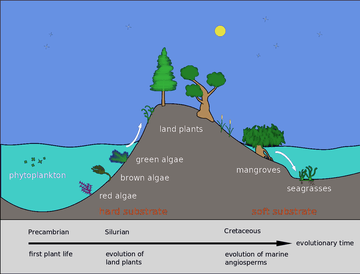
Back in the Silurian, some phytoplankton evolved into red, brown and green algae. These algae then invaded the land and started evolving into the land plants we know today. Later, in the Cretaceous, some of these land plants returned to the sea as marine plants, such as mangroves and seagrasses.[386]
Marine plants can be found in intertidal zones and shallow waters, such as seagrasses like eelgrass and turtle grass, Thalassia. These plants have adapted to the high salinity of the ocean environment. Plant life can also flourish in the brackish waters of estuaries, where mangroves or cordgrass or beach grass beach grass might grow.

 Seagrass meadow
Seagrass meadow Sea dragons camouflaged to look like floating seaweed live in kelp forests and seagrass meadows[387]
Sea dragons camouflaged to look like floating seaweed live in kelp forests and seagrass meadows[387]
The total world area of mangrove forests was estimated in 2010 as 134,257 square kilometres (51,837 sq mi) (based on satellite data).[388][389] The total world area of seagrass meadows is more difficult to determine, but was conservatively estimated in 2003 as 177,000 square kilometres (68,000 sq mi).[390]
Mangroves and seagrasses provide important nursery habitats for marine life, acting as hiding and foraging places for larval and juvenile forms of larger fish and invertebrates.
- Spalding, M. (2010) World atlas of mangroves, Routledge. ISBN 9781849776608. doi:10.4324/9781849776608.
Plankton and trophic interactions
Plankton (from Greek for wanderers) are a diverse group of organisms that live in the water column of large bodies of water but cannot swim against a current. As a result, they wander or drift with the currents.[391] Plankton are defined by their ecological niche, not by any phylogenetic or taxonomic classification. They are a crucial source of food for many marine animals, from forage fish to whales. Plankton can be divided into a plant-like component and an animal component.
Phytoplankton
Phytoplankton are the plant-like components of the plankton community ("phyto" comes from the Greek for plant). They are autotrophic (self-feeding), meaning they generate their own food and do not need to consume other organisms.
Phytoplankton consist mainly of microscopic photosynthetic eukaryotes which inhabit the upper sunlit layer in all oceans. They need sunlight so they can photosynthesize. Most phytoplankton are single-celled algae, but other phytoplankton are bacteria and some are protists.[392] Phytoplankton can be categorised into cyanobacteria (above), diatoms, various other types of algae (red, green, brown, and yellow-green), dinoflagellates, euglenoids, coccolithophorids, cryptomonads, chrysophytes, chlorophytes, prasinophytes, and silicoflagellates. They form the base of the primary production that drives the ocean food web, and account for half of the current global primary production, more than the terrestrial forests.[393]

- Phytoplankton
 Phytoplankton are the foundation of the ocean food chain
Phytoplankton are the foundation of the ocean food chain They come in many shapes and sizes.
They come in many shapes and sizes._Various_diatoms.jpg) Diatoms are one of the most common types of phytoplankton
Diatoms are one of the most common types of phytoplankton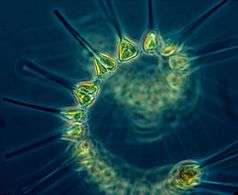 Colonial phytoplankton
Colonial phytoplankton
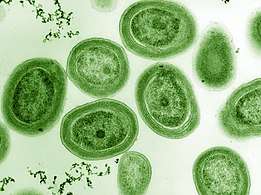 The cyanobacterium Prochlorococcus accounts for much of the ocean's primary production
The cyanobacterium Prochlorococcus accounts for much of the ocean's primary production- Green cyanobacteria scum washed up on a rock in California
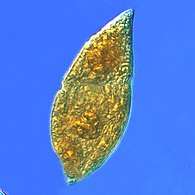 Gyrodinium, one of the few naked dinoflagellates which lack armour
Gyrodinium, one of the few naked dinoflagellates which lack armour.jpg) Zoochlorellae (green) living inside the ciliate Stichotricha secunda
Zoochlorellae (green) living inside the ciliate Stichotricha secunda
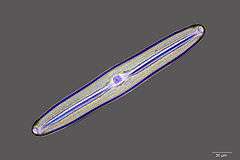
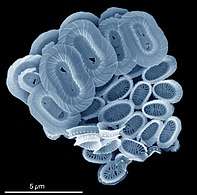 Coccolithophores named after the BBC documentary series
Coccolithophores named after the BBC documentary series
The Blue Planet.png) The coccolithophore Emiliania huxleyi
The coccolithophore Emiliania huxleyi Algae bloom of Emiliania huxleyi off the southern coast of England
Algae bloom of Emiliania huxleyi off the southern coast of England Guinardia delicatula, a diatom responsible for algal blooms in the North Sea and the English Channel [395]
Guinardia delicatula, a diatom responsible for algal blooms in the North Sea and the English Channel [395]
Zooplankton
Zooplankton are the animal component of the planktonic community ("zoo" comes from the Greek for animal). They are heterotrophic (other-feeding), meaning they cannot produce their own food and must consume instead other plants or animals as food. In particular, this means they eat phytoplankton.


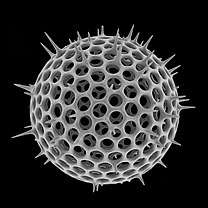
.jpg)
closely replicate some radiolarian shell patterns [396]
Zooplankton are generally larger than phytoplankton, mostly still microscopic but some can be seen with the naked eye. Many protozoans (single-celled protists that prey on other microscopic life) are zooplankton, including zooflagellates, foraminiferans, radiolarians and some dinoflagellates. Other dinoflagellates are mixotrophic and could also be classified as phytoplankton; the distinction between plants and animals often breaks down in very small organisms. Other zooplankton include pelagic cnidarians, ctenophores, molluscs, arthropods and tunicates, as well as planktonic arrow worms and bristle worms.
Radiolarians are unicellular protists with elaborate silica shells
Microzooplankton: major grazers of the plankton...
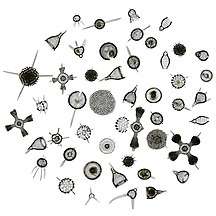 Radiolarians come in many shapes
Radiolarians come in many shapes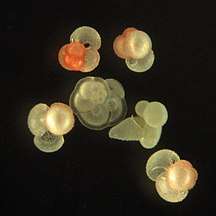 Group of planktic foraminiferans
Group of planktic foraminiferans Copepods eat phytoplankton. This one is carrying eggs.
Copepods eat phytoplankton. This one is carrying eggs.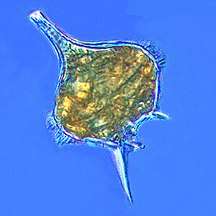 The dinoflagellate, Protoperidinium extrudes a large feeding veil to capture prey
The dinoflagellate, Protoperidinium extrudes a large feeding veil to capture prey
Larger zooplankton can be predatory on smaller zooplankton.
Macrozooplankton...
_Luc_Viatour.jpg) Moon jellyfish
Moon jellyfish Venus girdle, a ctenophore
Venus girdle, a ctenophore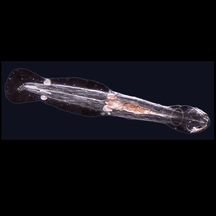 Arrow worm
Arrow worm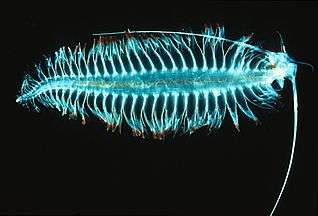 Tomopteris, a planktonic segmented worm with unusual yellow bioluminescence[397]
Tomopteris, a planktonic segmented worm with unusual yellow bioluminescence[397] Marine amphipod
Marine amphipod.jpg)
 Pelagic sea cucumber
Pelagic sea cucumber
Many marine animals begin life as zooplankton in the form of eggs or larvae, before they develop into adults. These are meroplanktic, that is, they are planktonic for only part of their life.
- Larvae and juveniles
 Salmon larva hatching from its egg
Salmon larva hatching from its egg Ocean sunfish larva
Ocean sunfish larva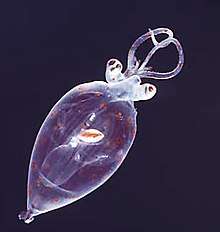 Juvenile planktonic squid
Juvenile planktonic squid Larva stage of a spiny lobster
Larva stage of a spiny lobster
Mixotrophic plankton

.jpg)
Dinoflagellates are often mixotrophic or live in symbiosis with other organisms.
- Mixoplankton
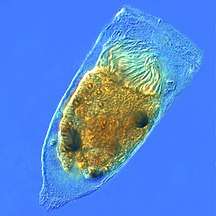 Tintinnid ciliate Favella
Tintinnid ciliate Favella_(cropped).jpg) Euglena mutabilis, a photosynthetic flagellate
Euglena mutabilis, a photosynthetic flagellate Noctiluca scintillans, a bioluminescence dinoflagellate
Noctiluca scintillans, a bioluminescence dinoflagellate
Some dinoflagellates are bioluminescent. At night, ocean water can light up internally and sparkle with blue light because of these dinoflagellates.[399][400] Bioluminescent dinoflagellates possess scintillons, individual cytoplasmic bodies which contain dinoflagellate luciferase, the main enzyme involved in the luminescence. The luminescence, sometimes called the phosphorescence of the sea, occurs as brief (0.1 sec) blue flashes or sparks when individual scintillons are stimulated, usually by mechanical disturbances from, for example, a boat or a swimmer or surf.[401]
Marine food web
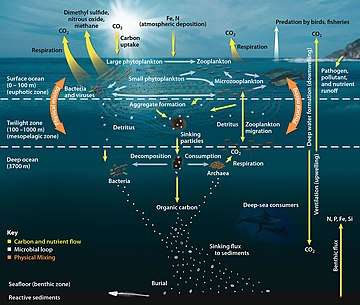
Compared to terrestrial environments, marine environments have biomass pyramids which are inverted at the base. In particular, the biomass of consumers (copepods, krill, shrimp, forage fish) is larger than the biomass of primary producers. This happens because the ocean's primary producers are tiny phytoplankton which tend to be r-strategists that grow and reproduce rapidly, so a small mass can have a fast rate of primary production. In contrast, terrestrial primary producers, such as mature forests, are often K-strategists that grow and reproduce slowly, so a much larger mass is needed to achieve the same rate of primary production.
Because of this inversion, it is the zooplankton that make up most of the marine animal biomass. As primary consumers, they are the crucial link between the primary producers (mainly phytoplankton) and the rest of the marine food web (secondary consumers).[402]
If phytoplankton dies before it is eaten, it descends through the euphotic zone as part of the marine snow and settles into the depths of sea. In this way, phytoplankton sequester about 2 billion tons of carbon dioxide into the ocean each year, causing the ocean to become a sink of carbon dioxide holding about 90% of all sequestered carbon.[403]
In 2010 researchers found whales carry nutrients from the depths of the ocean back to the surface using a process they called the whale pump.[404] Whales feed at deeper levels in the ocean where krill is found, but return regularly to the surface to breathe. There whales defecate a liquid rich in nitrogen and iron. Instead of sinking, the liquid stays at the surface where phytoplankton consume it. In the Gulf of Maine the whale pump provides more nitrogen than the rivers.[405]
Sediments and biogenic ooze
.jpg)
Sediments at the bottom of the ocean have two main origins, terrigenous and biogenous. Terrigenous sediments account for about 45% of the total marine sediment, and originate in the erosion of rocks on land, transported by rivers and land runoff, windborne dust, volcanoes, or grinding by glaciers.
Biogenous sediments account for the other 55% of the total sediment, and originate in the skeletal remains of marine protists (single-celled plankton and benthos organisms). Much smaller amounts of precipitated minerals and meteoric dust can also be present. Ooze, in the context of a marine sediment, does not refer to the consistency of the sediment but to its biological origin. The term ooze was originally used by John Murray, the "father of modern oceanography", who proposed the term radiolarian ooze for the silica deposits of radiolarian shells brought to the surface during the Challenger Expedition.[406] A biogenic ooze is a pelagic sediment containing at least 30 percent from the skeletal remains of marine organisms.
Main types of biogenic ooze | |||||||
|---|---|---|---|---|---|---|---|
| type | mineral forms |
protist responsible |
name of skeleton |
description | |||
| Siliceous ooze | SiO2 quartz glass opal chert |
diatoms |  |
frustule | Individual diatoms range in size from 0.002 to 0.2 mm.[407] | ||
| radiolarians | .jpg) |
skeleton | Radiolarians are protozoa with diameters between 0.1 and 0.2 mm that produce intricate mineral skeletons, usually made of silica | ||||
| Calcareous ooze | CaCO3 calcite aragonite limestone chalk |
foraminiferans |  |
test | There are about 10,000 living species of foraminiferans,[408] usually under 1 mm in size. | ||
| coccolithophores |  |
coccolith | Coccolithophores are spherical cells usually less than 0.1 mm across, enclosed by calcareous plates called coccoliths.[409] Coccoliths are important microfossils. They are the largest global source of biogenic calcium carbonate, and make significant contributions to the global carbon cycle.[410] They are the main constituent of chalk deposits such as the white cliffs of Dover. | ||||
%2C_Haeckel_(28187768550).jpg) An elaborate mineral skeleton of a radiolarian made of silica
An elaborate mineral skeleton of a radiolarian made of silica Diatoms, major components of marine plankton, also have silica skeletons called frustules
Diatoms, major components of marine plankton, also have silica skeletons called frustules Coccolithophores have plates or scales made with calcium carbonate called coccoliths
Coccolithophores have plates or scales made with calcium carbonate called coccoliths Calcified test of a planktic foraminiferan`
Calcified test of a planktic foraminiferan`
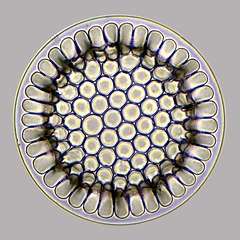 A diatom microfossil from 40 million years ago
A diatom microfossil from 40 million years ago Diatomaceous earth is a soft, siliceous, sedimentary rock made up of microfossils in the form of the frustules (shells) of single cell diatoms (click to magnify)
Diatomaceous earth is a soft, siliceous, sedimentary rock made up of microfossils in the form of the frustules (shells) of single cell diatoms (click to magnify) Illustration of a Globigerina ooze
Illustration of a Globigerina ooze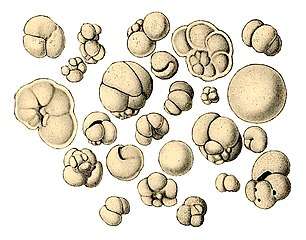 Shells (tests), usually made of calcium carbonate, from a foraminiferal ooze on the deep ocean floor
Shells (tests), usually made of calcium carbonate, from a foraminiferal ooze on the deep ocean floor
Biogeochemical cycles
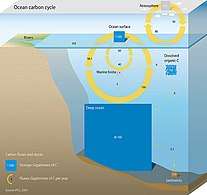 Marine carbon cycle[411]
Marine carbon cycle[411]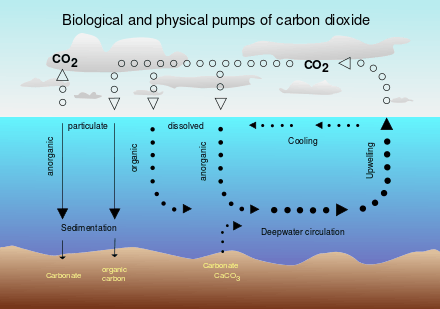 Biological pump
Biological pump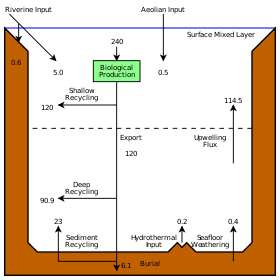 Marine silicon cycle
Marine silicon cycle- Rock cycle
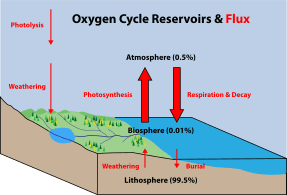 Oxygen cycle
Oxygen cycle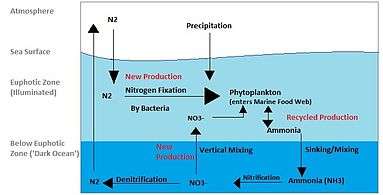
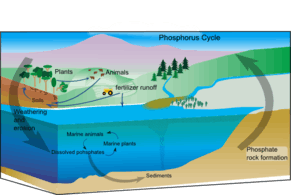 Marine phosphorus cycle
Marine phosphorus cycle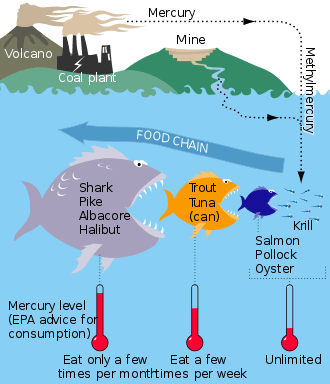 Mercury cycle
Mercury cycle
Land interactions
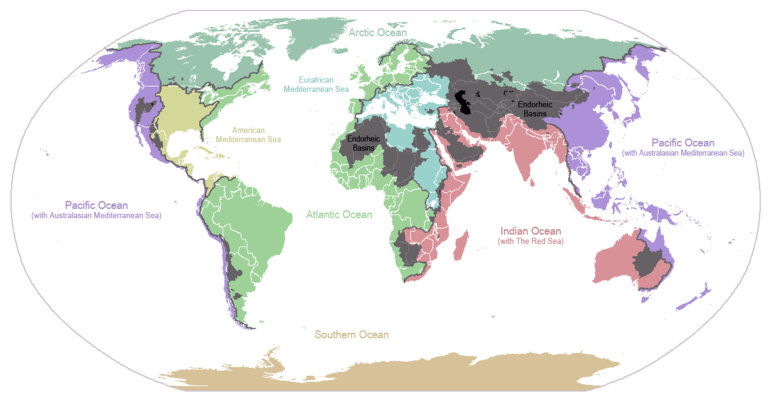
Land interactions impact marine life in many ways. Coastlines typically have continental shelves extending some way from the shore. These provide extensive shallows sunlit down to the seafloor, allowing for photosynthesis and enabling habitats for seagrass meadows, coral reefs, kelp forests and other benthic life. Further from shore the continental shelf slopes towards deep water. Wind blowing at the ocean surface or deep ocean currents can result in cold and nutrient rich waters from abyssal depths moving up the continental slopes. This can result in upwellings along the outer edges of continental shelves, providing conditions for phytoplankton blooms.
Water evaporated by the sun from the surface of the ocean can precipitate on land and eventually return to the ocean as runoff or discharge from rivers, enriched with nutrients as well as pollutants. As rivers discharge into estuaries, freshwater mixes with saltwater and becomes brackish. This provides another shallow water habitat where mangrove forests and estuarine fish thrive. Overall, life in inland lakes can evolve with greater diversity than happens in the sea, because freshwater habitats are themselves diverse and compartmentalised in a way marine habitats are not. Some aquatic life, such as salmon and eels, migrate back and forth between freshwater and marine habitats. These migrations can result in exchanges of pathogens and have impacts on the way life evolves in the ocean.
Anthropogenic impacts
Human activities affect marine life and marine habitats through overfishing, pollution, acidification and the introduction of invasive species. These impact marine ecosystems and food webs and may result in consequences as yet unrecognised for the biodiversity and continuation of marine life forms.[413]
Biodiversity and extinction events
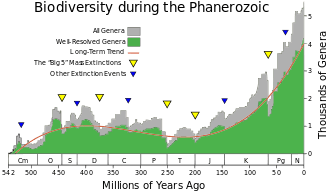
Biodiversity is the result of over three billion years of evolution. Until approximately 600 million years ago, all life consisted of archaea, bacteria, protozoans and similar single-celled organisms. The history of biodiversity during the Phanerozoic (the last 540 million years), starts with rapid growth during the Cambrian explosion – a period during which nearly every phylum of multicellular organisms first appeared. Over the next 400 million years or so, invertebrate diversity showed little overall trend and vertebrate diversity shows an overall exponential trend.[415]
However, more than 99 percent of all species that ever lived on Earth, amounting to over five billion species,[416] are estimated to be extinct.[417][418] These extinctions occur at an uneven rate. The dramatic rise in diversity has been marked by periodic, massive losses of diversity classified as mass extinction events.[415] Mass extinction events occur when life undergoes precipitous global declines. Most diversity and biomass on earth is found among the microorganisms, which are difficult to measure. Recorded extinction events are therefore based on the more easily observed changes in the diversity and abundance of larger multicellular organisms, rather than the total diversity and abundance of life.[419] Marine fossils are mostly used to measure extinction rates because of their superior fossil record and stratigraphic range compared to land organisms.
Based on the fossil record, the background rate of extinctions on Earth is about two to five taxonomic families of marine animals every million years. The Great Oxygenation Event was perhaps the first major extinction event. Since the Cambrian explosion five further major mass extinctions have significantly exceeded the background extinction rate.[420] The worst was the Permian-Triassic extinction event, 251 million years ago. Vertebrates took 30 million years to recover from this event.[421] In addition to these major mass extinctions there are numerous minor ones, as well as the current ongoing mass-extinction caused by human activity, the Holocene extinction sometimes called the "sixth extinction".
Marine biology

During the sixth century BC, the Greek philosopher Xenophanes (570-475 BC) recognised that some fossil shells were remains of shellfish. He used this to argue that what was at the time dry land was once under the sea.[422] This was an important step in advancing from simply stating an idea to backing it with evidence and observation.[423]
Later, during the fourth century BC, another Greek philosopher Aristotle (384–322 BC) attempted a comprehensive classification of animals which included systematic descriptions of many marine species,[424][425] and particularly species found in the Mediterranean Sea.[426] These pioneering works include History of Animals, a general biology of animals, Parts of Animals, a comparative anatomy and physiology of animals, and Generation of Animals, on developmental biology. The most striking passages are about the sea-life visible from observation on Lesbos and available from the catches of fishermen. His observations on catfish, electric fish (Torpedo) and angler-fish are detailed, as is his writing on cephalopods, namely, Octopus, Sepia (cuttlefish) and the paper nautilus (Argonauta argo). His description of the hectocotyl arm, used in sexual reproduction, was widely disbelieved until its rediscovery in the 19th century. He separated aquatic mammals from fish, and knew that sharks and rays were part of a group he called Selachē (selachians).[427] He gave accurate descriptions of the ovoviviparous embryological development of the hound shark Mustelus mustelus.[428] His classification of living things contains elements which were still in use in the 19th century. What the modern zoologist would call vertebrates and invertebrates, Aristotle called "animals with blood" and "animals without blood" (he did not know that complex invertebrates do make use of hemoglobin, but of a different kind from vertebrates). He divided animals with blood into live-bearing (mammals), and egg-bearing (birds and fish). Invertebrates ("animals without blood") he divided into insects, crustacea (further divided into non-shelled – cephalopods – and shelled) and testacea (molluscs).[429][430]
In contemporary times, marine life is a field of study both in marine biology and in biological oceanography. In biology many phyla, families and genera have some species that live in the sea and others that live on land. Marine biology classifies species based on the environment rather than on taxonomy. For this reason marine biology encompasses not only organisms that live only in a marine environment, but also other organisms whose lives revolve around the sea. Biological oceanography is the study of how organisms affect and are affected by the physics, chemistry, and geology of the oceanographic system. Biological oceanography mostly focuses on the microorganisms within the ocean; looking at how they are affected by their environment and how that affects larger marine creatures and their ecosystem.[431] Biological oceanography is similar to marine biology, but it studies ocean life from a different perspective. Biological oceanography takes a bottom up approach in terms of the food web, while marine biology studies the ocean from a top down perspective. Biological oceanography mainly focuses on the ecosystem of the ocean with an emphasis on plankton: their diversity (morphology, nutritional sources, motility, and metabolism); their productivity and how that plays a role in the global carbon cycle; and their distribution (predation and life cycle).[431][432][433] Biological oceanography also investigates the role of microbes in food webs, and how humans impact the ecosystems in the oceans.[431]
See also
- Blue Planet – British nature documentary series - David Attenborough
- Blue Planet II
- Census of Marine Life
- Colonization of land
- Taxonomy of invertebrates – System of classification of animals with emphasis on the invertebrates
Notes
- This is the measurement taken by the vessel Kaikō in March 1995 and is considered the most accurate measurement to date. See the Challenger Deep article for more details.
- Myxozoa were thought to be an exception, but are now thought to be heavily modified members of the Cnidaria. Jímenez-Guri, Eva; Philippe, Hervé; Okamura, Beth; Holland, Peter W. H. (6 July 2007). "Buddenbrockia Is a Cnidarian Worm". Science. 317 (5834): 116–118. Bibcode:2007Sci...317..116J. doi:10.1126/science.1142024. ISSN 0036-8075. PMID 17615357.CS1 maint: ref=harv (link)
References
- "National Oceanic and Atmospheric Administration – Ocean". NOAA. Retrieved 20 February 2019.
- Tiny Fish May Be Ancestor of Nearly All Living Vertebrates Live Science, 11 June 2014.
- Drogin, Bob (2 August 2009). "Mapping an ocean of species". Los Angeles Times. Retrieved 18 August 2009.
- Paul, Gregory S. (2010). "The Evolution of Dinosaurs and their World". The Princeton Field Guide to Dinosaurs. Princeton: Princeton University Press. p. 19.
- Bortolotti, Dan (2008). Wild Blue: A Natural History of the World's Largest Animal. St. Martin's Press.
- Xiao-feng, Pang (2014) Water: Molecular Structure And Properties, chapter 5, pp. 390–461, World Scientific. ISBN 9789814440448
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 620. ISBN 978-0-08-037941-8.
- "Water, the Universal Solvent". USGS. Archived from the original on 9 July 2017. Retrieved 27 June 2017.
- Reece, Jane B. (31 October 2013). Campbell Biology (10 ed.). Pearson. p. 48. ISBN 9780321775658.
- Reece, Jane B. (31 October 2013). Campbell Biology (10 ed.). Pearson. p. 44. ISBN 9780321775658.
- Collins J. C. (1991) The Matrix of Life: A View of Natural Molecules from the Perspective of Environmental Water Molecular Presentations. ISBN 9780962971907.
- "7,000 m Class Remotely Operated Vehicle KAIKO 7000". Japan Agency for Marine-Earth Science and Technology (JAMSTEC). Retrieved 7 June 2008.
- Charette, Matthew A.; Smith, Walter H. F. (June 2010). "The Volume of Earth's Ocean" (PDF). Oceanography. 23 (2): 112–14. doi:10.5670/oceanog.2010.51. Archived from the original (PDF) on 2 August 2013. Retrieved 6 June 2013.
- "sphere depth of the ocean – hydrology". Encyclopædia Britannica. Retrieved 12 April 2015.
- "Third rock from the Sun – restless Earth". NASA's Cosmos. Retrieved 12 April 2015.
- Perlman, Howard (17 March 2014). "The World's Water". USGS Water-Science School. Retrieved 12 April 2015.
- Kennish, Michael J. (2001). Practical handbook of marine science. Marine science series (3rd ed.). CRC Press. p. 35. ISBN 978-0-8493-2391-1.
- "Why is the ocean salty?".
- Mullen, Leslie (11 June 2002). "Salt of the Early Earth". NASA Astrobiology Magazine. Archived from the original on 22 July 2007. Retrieved 14 March 2007.
- Morris, Ron M. "Oceanic Processes". NASA Astrobiology Magazine. Archived from the original on 15 April 2009. Retrieved 14 March 2007.
- Scott, Michon (24 April 2006). "Earth's Big heat Bucket". NASA Earth Observatory. Retrieved 14 March 2007.
- Sample, Sharron (21 June 2005). "Sea Surface Temperature". NASA. Archived from the original on 3 April 2013. Retrieved 21 April 2007.
- "Volumes of the World's Oceans from ETOPO1". NOAA. Archived from the original on 11 March 2015. Retrieved 20 February 2019.CS1 maint: BOT: original-url status unknown (link)
- Planet "Earth": We Should Have Called It "Sea" Quote Invertigator, 25 January 2017.
- Unveiling Planet Ocean NASA Science, 14 March 2002.
- Dyches, Preston; Brown, Dwayne (12 May 2015). "NASA Research Reveals Europa's Mystery Dark Material Could Be Sea Salt". NASA. Retrieved 12 May 2015.
- "Water near surface of a Jupiter moon only temporary".
- Tritt, Charles S. (2002). "Possibility of Life on Europa". Milwaukee School of Engineering. Archived from the original on 9 June 2007. Retrieved 10 August 2007.
- Schulze-Makuch, Dirk; Irwin, Louis N. (2001). "Alternative Energy Sources Could Support Life on Europa" (PDF). Departments of Geological and Biological Sciences, University of Texas at El Paso. Archived from the original (PDF) on 3 July 2006. Retrieved 21 December 2007.
- Friedman, Louis (14 December 2005). "Projects: Europa Mission Campaign". The Planetary Society. Archived from the original on 11 August 2011. Retrieved 8 August 2011.
- Ocean Within Enceladus May Harbor Hydrothermal Activity, NASA Press Release. 11 March 2015.
- "Age of the Earth". United States Geological Survey. 9 July 2007. Retrieved 31 May 2015.
- Dalrymple 2001, pp. 205–221
- Manhesa, Gérard; Allègre, Claude J.; Dupréa, Bernard; Hamelin, Bruno (May 1980). "Lead isotope study of basic-ultrabasic layered complexes: Speculations about the age of the earth and primitive mantle characteristics". Earth and Planetary Science Letters. 47 (3): 370–382. Bibcode:1980E&PSL..47..370M. doi:10.1016/0012-821X(80)90024-2. ISSN 0012-821X.
- Schopf, J. William; Kudryavtsev, Anatoliy B.; Czaja, Andrew D.; Tripathi, Abhishek B. (5 October 2007). "Evidence of Archean life: Stromatolites and microfossils". Precambrian Research. 158 (3–4): 141–155. Bibcode:2007PreR..158..141S. doi:10.1016/j.precamres.2007.04.009. ISSN 0301-9268.
- Raven & Johnson 2002, p. 68
- Baumgartner, R. J.; et al. (2019). "Nano−porous pyrite and organic matter in 3.5-billion-year-old stromatolites record primordial life". Geology. 47: 1039–1043. doi:10.1130/G46365.1.
- Earliest signs of life: Scientists find microbial remains in ancient rocks Phys.org. 26 September 2019.
- Ohtomo, Yoko; Kakegawa, Takeshi; Ishida, Akizumi; et al. (January 2014). "Evidence for biogenic graphite in early Archaean Isua metasedimentary rocks". Nature Geoscience. 7 (1): 25–28. Bibcode:2014NatGe...7...25O. doi:10.1038/ngeo2025. ISSN 1752-0894.
- Borenstein, Seth (19 October 2015). "Hints of life on what was thought to be desolate early Earth". Associated Press. Retrieved 9 October 2018.
- Bell, Elizabeth A.; Boehnike, Patrick; Harrison, T. Mark; et al. (24 November 2015). "Potentially biogenic carbon preserved in a 4.1 billion-year-old zircon" (PDF). Proceedings of the National Academy of Sciences of the United States of America. 112 (47): 14518–14521. Bibcode:2015PNAS..11214518B. doi:10.1073/pnas.1517557112. ISSN 0027-8424. PMC 4664351. PMID 26483481. Retrieved 30 December 2015.
- Penny, David; Poole, Anthony (December 1999). "The nature of the last universal common ancestor". Current Opinion in Genetics & Development. 9 (6): 672–677. doi:10.1016/S0959-437X(99)00020-9. ISSN 0959-437X. PMID 10607605.
- Theobald, Douglas L. (13 May 2010). "A formal test of the theory of universal common ancestry". Nature. 465 (7295): 219–222. Bibcode:2010Natur.465..219T. doi:10.1038/nature09014. ISSN 0028-0836. PMID 20463738.
- Doolittle, W. Ford (February 2000). "Uprooting the Tree of Life" (PDF). Scientific American. 282 (2): 90–95. Bibcode:2000SciAm.282b..90D. doi:10.1038/scientificamerican0200-90. ISSN 0036-8733. PMID 10710791. Archived from the original (PDF) on 7 September 2006. Retrieved 5 April 2015.
- Peretó, Juli (March 2005). "Controversies on the origin of life" (PDF). International Microbiology. 8 (1): 23–31. ISSN 1139-6709. PMID 15906258. Archived from the original (PDF) on 24 August 2015.
- Joyce, Gerald F. (11 July 2002). "The antiquity of RNA-based evolution". Nature. 418 (6894): 214–221. Bibcode:2002Natur.418..214J. doi:10.1038/418214a. ISSN 0028-0836. PMID 12110897.
- Trevors, Jack T.; Psenner, Roland (December 2001). "From self-assembly of life to present-day bacteria: a possible role for nanocells". FEMS Microbiology Reviews. 25 (5): 573–582. doi:10.1111/j.1574-6976.2001.tb00592.x. ISSN 1574-6976. PMID 11742692.
- Wade, Nicholas (25 July 2016). "Meet Luca, the Ancestor of All Living Things". New York Times. Retrieved 25 July 2016.
- Bapteste, Eric; Walsh, David A. (June 2005). "Does the 'Ring of Life' ring true?". Trends in Microbiology. 13 (6): 256–261. doi:10.1016/j.tim.2005.03.012. ISSN 0966-842X. PMID 15936656.
- Darwin 1859, p. 1
- Doolittle, W. Ford; Bapteste, Eric (13 February 2007). "Pattern pluralism and the Tree of Life hypothesis". Proceedings of the National Academy of Sciences of the United States of America. 104 (7): 2043–2049. Bibcode:2007PNAS..104.2043D. doi:10.1073/pnas.0610699104. ISSN 0027-8424. PMC 1892968. PMID 17261804.
- Kunin, Victor; Goldovsky, Leon; Darzentas, Nikos; Ouzounis, Christos A. (July 2005). "The net of life: Reconstructing the microbial phylogenetic network". Genome Research. 15 (7): 954–959. doi:10.1101/gr.3666505. ISSN 1088-9051. PMC 1172039. PMID 15965028.
- Jablonski, David (25 June 1999). "The Future of the Fossil Record". Science. 284 (5423): 2114–2116. doi:10.1126/science.284.5423.2114. ISSN 0036-8075. PMID 10381868.
- Mason, Stephen F. (6 September 1984). "Origins of biomolecular handedness". Nature. 311 (5981): 19–23. Bibcode:1984Natur.311...19M. doi:10.1038/311019a0. ISSN 0028-0836. PMID 6472461.
- Wolf, Yuri I.; Rogozin, Igor B.; Grishin, Nick V.; Koonin, Eugene V. (1 September 2002). "Genome trees and the tree of life". Trends in Genetics. 18 (9): 472–479. doi:10.1016/S0168-9525(02)02744-0. ISSN 0168-9525. PMID 12175808.
- Varki, Ajit; Altheide, Tasha K. (December 2005). "Comparing the human and chimpanzee genomes: searching for needles in a haystack" (PDF). Genome Research. 15 (12): 1746–1758. doi:10.1101/gr.3737405. ISSN 1088-9051. PMID 16339373.
- Cavalier-Smith, Thomas (29 June 2006). "Cell evolution and Earth history: stasis and revolution". Philosophical Transactions of the Royal Society B: Biological Sciences. 361 (1470): 969–1006. doi:10.1098/rstb.2006.1842. ISSN 0962-8436. PMC 1578732. PMID 16754610.
- Schopf, J. William (29 June 2006). "Fossil evidence of Archaean life". Philosophical Transactions of the Royal Society B: Biological Sciences. 361 (1470): 869–885. doi:10.1098/rstb.2006.1834. ISSN 0962-8436. PMC 1578735. PMID 16754604.
- Altermann, Wladyslaw; Kazmierczak, Józef (November 2003). "Archean microfossils: a reappraisal of early life on Earth". Research in Microbiology. 154 (9): 611–617. doi:10.1016/j.resmic.2003.08.006. ISSN 0923-2508. PMID 14596897.
- Schopf, J. William (19 July 1994). "Disparate rates, differing fates: tempo and mode of evolution changed from the Precambrian to the Phanerozoic". Proceedings of the National Academy of Sciences of the United States of America. 91 (15): 6735–6742. Bibcode:1994PNAS...91.6735S. doi:10.1073/pnas.91.15.6735. ISSN 0027-8424. PMC 44277. PMID 8041691.
- Poole, Anthony M.; Penny, David (January 2007). "Evaluating hypotheses for the origin of eukaryotes". BioEssays. 29 (1): 74–84. doi:10.1002/bies.20516. ISSN 0265-9247. PMID 17187354.
- Dyall, Sabrina D.; Brown, Mark T.; Johnson, Patricia J. (9 April 2004). "Ancient Invasions: From Endosymbionts to Organelles". Science. 304 (5668): 253–257. Bibcode:2004Sci...304..253D. doi:10.1126/science.1094884. ISSN 0036-8075. PMID 15073369.
- Martin, William (October 2005). "The missing link between hydrogenosomes and mitochondria". Trends in Microbiology. 13 (10): 457–459. doi:10.1016/j.tim.2005.08.005. ISSN 0966-842X. PMID 16109488.
- Lang, B. Franz; Gray, Michael W.; Burger, Gertraud (December 1999). "Mitochondrial genome evolution and the origin of eukaryotes". Annual Review of Genetics. 33: 351–397. doi:10.1146/annurev.genet.33.1.351. ISSN 0066-4197. PMID 10690412.
- McFadden, Geoffrey Ian (1 December 1999). "Endosymbiosis and evolution of the plant cell". Current Opinion in Plant Biology. 2 (6): 513–519. doi:10.1016/S1369-5266(99)00025-4. ISSN 1369-5266. PMID 10607659.
- DeLong, Edward F.; Pace, Norman R. (1 August 2001). "Environmental Diversity of Bacteria and Archaea". Systematic Biology. 50 (4): 470–478. CiteSeerX 10.1.1.321.8828. doi:10.1080/106351501750435040. ISSN 1063-5157. PMID 12116647.
- Kaiser, Dale (December 2001). "Building a multicellular organism". Annual Review of Genetics. 35: 103–123. doi:10.1146/annurev.genet.35.102401.090145. ISSN 0066-4197. PMID 11700279.
- Zimmer, Carl (7 January 2016). "Genetic Flip Helped Organisms Go From One Cell to Many". The New York Times. Retrieved 7 January 2016.
- Valentine, James W.; Jablonski, David; Erwin, Douglas H. (1 March 1999). "Fossils, molecules and embryos: new perspectives on the Cambrian explosion". Development. 126 (5): 851–859. ISSN 0950-1991. PMID 9927587. Retrieved 30 December 2014.
- Ohno, Susumu (January 1997). "The reason for as well as the consequence of the Cambrian explosion in animal evolution". Journal of Molecular Evolution. 44 (Suppl. 1): S23–S27. Bibcode:1997JMolE..44S..23O. doi:10.1007/PL00000055. ISSN 0022-2844. PMID 9071008.
- Valentine, James W.; Jablonski, David (2003). "Morphological and developmental macroevolution: a paleontological perspective". The International Journal of Developmental Biology. 47 (7–8): 517–522. ISSN 0214-6282. PMID 14756327. Retrieved 30 December 2014.
- Wellman, Charles H.; Osterloff, Peter L.; Mohiuddin, Uzma (2003). "Fragments of the earliest land plants" (PDF). Nature. 425 (6955): 282–285. Bibcode:2003Natur.425..282W. doi:10.1038/nature01884. PMID 13679913.
- Barton, Nicholas (2007). Evolution. pp. 273–274. ISBN 9780199226320. Retrieved 30 September 2012.
- Waters, Elizabeth R. (December 2003). "Molecular adaptation and the origin of land plants". Molecular Phylogenetics and Evolution. 29 (3): 456–463. doi:10.1016/j.ympev.2003.07.018. ISSN 1055-7903. PMID 14615186.
- Mayhew, Peter J. (August 2007). "Why are there so many insect species? Perspectives from fossils and phylogenies". Biological Reviews. 82 (3): 425–454. doi:10.1111/j.1469-185X.2007.00018.x. ISSN 1464-7931. PMID 17624962.
- Carroll, Robert L. (May 2007). "The Palaeozoic Ancestry of Salamanders, Frogs and Caecilians". Zoological Journal of the Linnean Society. 150 (Supplement s1): 1–140. doi:10.1111/j.1096-3642.2007.00246.x. ISSN 1096-3642.
- Wible, John R.; Rougier, Guillermo W.; Novacek, Michael J.; Asher, Robert J. (21 June 2007). "Cretaceous eutherians and Laurasian origin for placental mammals near the K/T boundary". Nature. 447 (7147): 1003–1006. Bibcode:2007Natur.447.1003W. doi:10.1038/nature05854. ISSN 0028-0836. PMID 17581585.
- Witmer, Lawrence M. (28 July 2011). "Palaeontology: An icon knocked from its perch". Nature. 475 (7357): 458–459. doi:10.1038/475458a. ISSN 0028-0836. PMID 21796198.
- Schloss, Patrick D.; Handelsman, Jo (December 2004). "Status of the Microbial Census". Microbiology and Molecular Biology Reviews. 68 (4): 686–691. doi:10.1128/MMBR.68.4.686-691.2004. ISSN 1092-2172. PMC 539005. PMID 15590780.
- Miller & Spoolman 2012, p. 62
- Mora, Camilo; Tittensor, Derek P.; Adl, Sina; et al. (23 August 2011). "How Many Species Are There on Earth and in the Ocean?". PLoS Biology. 9 (8): e1001127. doi:10.1371/journal.pbio.1001127. ISSN 1545-7885. PMC 3160336. PMID 21886479.
- Bar-On, YM; Phillips, R; Milo, R (2018). "The biomass distribution on Earth". PNAS. 115 (25): 6506–6511. doi:10.1073/pnas.1711842115. PMC 6016768. PMID 29784790.
- Madigan M; Martinko J, eds. (2006). Brock Biology of Microorganisms (13th ed.). Pearson Education. p. 1096. ISBN 978-0-321-73551-5.
- Rybicki EP (1990). "The classification of organisms at the edge of life, or problems with virus systematics". South African Journal of Science. 86: 182–6. ISSN 0038-2353.
- Lwoff A (1956). "The concept of virus". Journal of General Microbiology. 17 (2): 239–53. doi:10.1099/00221287-17-2-239. PMID 13481308.
- 2002 WHO mortality data Accessed 20 January 2007
- "Functions of global ocean microbiome key to understanding environmental changes". www.sciencedaily.com. University of Georgia. 10 December 2015. Retrieved 11 December 2015.
- Suttle, C.A. (2005). "Viruses in the Sea". Nature. 437 (9): 356–361. Bibcode:2005Natur.437..356S. doi:10.1038/nature04160. PMID 16163346.
- Shors p. 5
- Shors p. 593
- Suttle CA. Marine viruses—major players in the global ecosystem. Nature Reviews Microbiology. 2007;5(10):801–12. doi:10.1038/nrmicro1750. PMID 17853907.
- Living Bacteria Are Riding Earth’s Air Currents Smithsonian Magazine, 11 January 2016.
- Robbins, Jim (13 April 2018). "Trillions Upon Trillions of Viruses Fall From the Sky Each Day". The New York Times. Retrieved 14 April 2018.
- Reche, Isabel; D’Orta, Gaetano; Mladenov, Natalie; Winget, Danielle M; Suttle, Curtis A (29 January 2018). "Deposition rates of viruses and bacteria above the atmospheric boundary layer". ISME Journal. 12 (4): 1154–1162. doi:10.1038/s41396-017-0042-4. PMC 5864199. PMID 29379178.
- Staff (2014). "The Biosphere". Aspen Global Change Institute. Retrieved 10 November 2014.
- Choi, Charles Q. (17 March 2013). "Microbes Thrive in Deepest Spot on Earth". LiveScience. Retrieved 17 March 2013.
- Glud, Ronnie; Wenzhöfer, Frank; Middelboe, Mathias; Oguri, Kazumasa; Turnewitsch, Robert; Canfield, Donald E.; Kitazato, Hiroshi (17 March 2013). "High rates of microbial carbon turnover in sediments in the deepest oceanic trench on Earth". Nature Geoscience. 6 (4): 284–288. Bibcode:2013NatGe...6..284G. doi:10.1038/ngeo1773.
- Oskin, Becky (14 March 2013). "Intraterrestrials: Life Thrives in Ocean Floor". LiveScience. Retrieved 17 March 2013.
- Morelle, Rebecca (15 December 2014). "Microbes discovered by deepest marine drill analysed". BBC News. Retrieved 15 December 2014.
- Takai K; Nakamura K; Toki T; Tsunogai U; et al. (2008). "Cell proliferation at 122°C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high-pressure cultivation". Proceedings of the National Academy of Sciences of the United States of America. 105 (31): 10949–54. Bibcode:2008PNAS..10510949T. doi:10.1073/pnas.0712334105. PMC 2490668. PMID 18664583.
- Fox, Douglas (20 August 2014). "Lakes under the ice: Antarctica's secret garden". Nature. 512 (7514): 244–246. Bibcode:2014Natur.512..244F. doi:10.1038/512244a. PMID 25143097.
- Mack, Eric (20 August 2014). "Life Confirmed Under Antarctic Ice; Is Space Next?". Forbes. Retrieved 21 August 2014.
- Wimmer E, Mueller S, Tumpey TM, Taubenberger JK. Synthetic viruses: a new opportunity to understand and prevent viral disease. Nature Biotechnology. 2009;27(12):1163–72. doi:10.1038/nbt.1593. PMID 20010599.
- Koonin EV, Senkevich TG, Dolja VV. The ancient Virus World and evolution of cells. Biology Direct. 2006;1:29. doi:10.1186/1745-6150-1-29. PMID 16984643.
- Collier, Leslie; Balows, Albert; Sussman, Max (1998) Topley and Wilson's Microbiology and Microbial Infections ninth edition, Volume 1, Virology, volume editors: Mahy, Brian and Collier, Leslie. Arnold. Pages 33–37. ISBN 0-340-66316-2.
- Iyer LM, Balaji S, Koonin EV, Aravind L. Evolutionary genomics of nucleo-cytoplasmic large DNA viruses. Virus Research. 2006;117(1):156–84. doi:10.1016/j.virusres.2006.01.009. PMID 16494962.
- Sanjuán R, Nebot MR, Chirico N, Mansky LM, Belshaw R. Viral mutation rates. Journal of Virology. 2010;84(19):9733–48. doi:10.1128/JVI.00694-10. PMID 20660197.
- In: Mahy WJ, Van Regenmortel MHV. Desk Encyclopedia of General Virology. Oxford: Academic Press; 2009. ISBN 0-12-375146-2. p. 28.
- Canchaya C, Fournous G, Chibani-Chennoufi S, Dillmann ML, Brüssow H. Phage as agents of lateral gene transfer. Current Opinion in Microbiology. 2003;6(4):417–24. doi:10.1016/S1369-5274(03)00086-9. PMID 12941415.
- Are viruses alive? The replicator paradigm sheds decisive light on an old but misguided question. Studies in History and Philosophy of Biological and Biomedical Sciences. 2016. doi:10.1016/j.shpsc.2016.02.016. PMID 26965225.
- Koonin, E. V.; Starokadomskyy, P. (7 March 2016). "Are viruses alive? The replicator paradigm sheds decisive light on an old but misguided question". Studies in History and Philosophy of Biological and Biomedical Sciences. 59: 125–34. doi:10.1016/j.shpsc.2016.02.016. PMC 5406846. PMID 26965225.
- Rybicki, EP. The classification of organisms at the edge of life, or problems with virus systematics. South African Journal of Science. 1990;86:182–186.
- Mann, NH (17 May 2005). "The Third Age of Phage". PLoS Biology. 3 (5): 753–755. doi:10.1371/journal.pbio.0030182. PMC 1110918. PMID 15884981.
- Wommack KE, Colwell RR. Virioplankton: viruses in aquatic ecosystems. Microbiology and Molecular Biology Reviews. 2000;64(1):69–114. doi:10.1128/MMBR.64.1.69-114.2000. PMID 10704475.
- Suttle CA. Viruses in the sea. Nature. 2005;437:356–361. doi:10.1038/nature04160. PMID 16163346.
- Bergh O, Børsheim KY, Bratbak G, Heldal M. High abundance of viruses found in aquatic environments. Nature. 1989;340(6233):467–8. doi:10.1038/340467a0. PMID 2755508. Bibcode: 1989Natur.340..467B.
- Wigington CH, Sonderegger D, Brussaard CPD, Buchan A, Finke JF, Fuhrman JA, Lennon JT, Middelboe M, Suttle CA, Stock C, Wilson WH, Wommack KE, Wilhelm SW, Weitz JS. Re-examination of the relationship between marine virus and microbial cell abundances. Nature Microbiology. 2016;1:15024. doi:10.1038/nmicrobiol.2015.24. PMID 27572161.
- Krupovic M, Bamford DH (2007). "Putative prophages related to lytic tailless marine dsDNA phage PM2 are widespread in the genomes of aquatic bacteria". BMC Genomics. 8: 236. doi:10.1186/1471-2164-8-236. PMC 1950889. PMID 17634101.
- Xue H, Xu Y, Boucher Y, Polz MF (2012). "High frequency of a novel filamentous phage, VCY φ, within an environmental Vibrio cholerae population". Applied and Environmental Microbiology. 78 (1): 28–33. doi:10.1128/AEM.06297-11. PMC 3255608. PMID 22020507.
- Roux S, Krupovic M, Poulet A, Debroas D, Enault F (2012). "Evolution and diversity of the Microviridae viral family through a collection of 81 new complete genomes assembled from virome reads". PLOS ONE. 7 (7): e40418. Bibcode:2012PLoSO...740418R. doi:10.1371/journal.pone.0040418. PMC 3394797. PMID 22808158.
- Suttle CA. Viruses in the sea. Nature. 2005;437(7057):356–61. doi:10.1038/nature04160. PMID 16163346. Bibcode: 2005Natur.437..356S.
- www.cdc.gov. Harmful Algal Blooms: Red Tide: Home [Retrieved 2014-12-19].
- Lawrence CM, Menon S, Eilers BJ, et al.. Structural and functional studies of archaeal viruses. Journal of Biological Chemistry. 2009;284(19):12599–603. doi:10.1074/jbc.R800078200. PMID 19158076.
- Prangishvili D, Forterre P, Garrett RA. Viruses of the Archaea: a unifying view. Nature Reviews Microbiology. 2006;4(11):837–48. doi:10.1038/nrmicro1527. PMID 17041631.
- Prangishvili D, Garrett RA. Exceptionally diverse morphotypes and genomes of crenarchaeal hyperthermophilic viruses. Biochemical Society Transactions. 2004;32(Pt 2):204–8. doi:10.1042/BST0320204. PMID 15046572.
- Forterre P, Philippe H. The last universal common ancestor (LUCA), simple or complex?. The Biological Bulletin. 1999;196(3):373–5; discussion 375–7. doi:10.2307/1542973. PMID 11536914.
- Fredrickson JK, Zachara JM, Balkwill DL, Kennedy D, Li SM, Kostandarithes HM, Daly MJ, Romine MF, Brockman FJ (2004). "Geomicrobiology of high-level nuclear waste-contaminated vadose sediments at the Hanford site, Washington state". Applied and Environmental Microbiology. 70 (7): 4230–41. doi:10.1128/AEM.70.7.4230-4241.2004. PMC 444790. PMID 15240306.
- Woese CR, Kandler O, Wheelis ML (1990). "Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya". Proceedings of the National Academy of Sciences of the United States of America. 87 (12): 4576–9. Bibcode:1990PNAS...87.4576W. doi:10.1073/pnas.87.12.4576. PMC 54159. PMID 2112744.
- Schopf JW (1994). "Disparate rates, differing fates: tempo and mode of evolution changed from the Precambrian to the Phanerozoic". Proceedings of the National Academy of Sciences of the United States of America. 91 (15): 6735–42. Bibcode:1994PNAS...91.6735S. doi:10.1073/pnas.91.15.6735. PMC 44277. PMID 8041691.
- DeLong EF, Pace NR (2001). "Environmental diversity of bacteria and archaea". Systematic Biology. 50 (4): 470–8. CiteSeerX 10.1.1.321.8828. doi:10.1080/106351501750435040. PMID 12116647.
- Brown JR, Doolittle WF (1997). "Archaea and the prokaryote-to-eukaryote transition". Microbiology and Molecular Biology Reviews. 61 (4): 456–502. doi:10.1128/.61.4.456-502.1997. PMC 232621. PMID 9409149.
- Poole AM, Penny D (2007). "Evaluating hypotheses for the origin of eukaryotes". BioEssays. 29 (1): 74–84. doi:10.1002/bies.20516. PMID 17187354.
- Lang BF, Gray MW, Burger G (1999). "Mitochondrial genome evolution and the origin of eukaryotes". Annual Review of Genetics. 33: 351–97. doi:10.1146/annurev.genet.33.1.351. PMID 10690412.
- McFadden GI (1999). "Endosymbiosis and evolution of the plant cell". Current Opinion in Plant Biology. 2 (6): 513–9. doi:10.1016/S1369-5266(99)00025-4. PMID 10607659.
- Patrick J. Keeling (2004). "Diversity and evolutionary history of plastids and their hosts". American Journal of Botany. 91 (10): 1481–1493. doi:10.3732/ajb.91.10.1481. PMID 21652304.
- "The largest Bacterium: Scientist discovers new bacterial life form off the African coast", Max Planck Institute for Marine Microbiology, 8 April 1999, archived from the original on 20 January 2010
- List of Prokaryotic names with Standing in Nomenclature - Genus Thiomargarita
- Bang C, Schmitz RA (2015). "Archaea associated with human surfaces: not to be underestimated". FEMS Microbiology Reviews. 39 (5): 631–48. doi:10.1093/femsre/fuv010. PMID 25907112.
- Archaea Online Etymology Dictionary. Retrieved 17 August 2016.
- Pace NR (May 2006). "Time for a change". Nature. 441 (7091): 289. Bibcode:2006Natur.441..289P. doi:10.1038/441289a. PMID 16710401.
- Stoeckenius W (1 October 1981). "Walsby's square bacterium: fine structure of an orthogonal procaryote". Journal of Bacteriology. 148 (1): 352–60. doi:10.1128/JB.148.1.352-360.1981. PMC 216199. PMID 7287626.
- Whittaker, R.H.; Margulis, L. (1978). "Protist classification and the kingdoms of organisms". Biosystems. 10 (1–2): 3–18. doi:10.1016/0303-2647(78)90023-0. PMID 418827.
- Faure, E; Not, F; Benoiston, AS; Labadie, K; Bittner, L; Ayata, SD (2019). "Mixotrophic protists display contrasted biogeographies in the global ocean" (PDF). ISME Journal. 13 (4): 1072–1083. doi:10.1038/s41396-018-0340-5. PMC 6461780. PMID 30643201.
- Leles, S.G.; Mitra, A.; Flynn, K.J.; Stoecker, D.K.; Hansen, P.J.; Calbet, A.; McManus, G.B.; Sanders, R.W.; Caron, D.A.; Not, F.; Hallegraeff, G.M. (1860). "Oceanic protists with different forms of acquired phototrophy display contrasting biogeographies and abundance". Proceedings of the Royal Society B: Biological Sciences. 284 (1860): 20170664. doi:10.1098/rspb.2017.0664. PMC 5563798. PMID 28768886.
- Budd, Graham E; Jensen, Sören (2017). "The origin of the animals and a 'Savannah' hypothesis for early bilaterian evolution". Biological Reviews. 92 (1): 446–473. doi:10.1111/brv.12239. PMID 26588818.
- Cavalier-Smith T (December 1993). "Kingdom protozoa and its 18 phyla". Microbiological Reviews. 57 (4): 953–94. doi:10.1128/MMBR.57.4.953-994.1993. PMC 372943. PMID 8302218.
- Corliss JO (1992). "Should there be a separate code of nomenclature for the protists?". BioSystems. 28 (1–3): 1–14. doi:10.1016/0303-2647(92)90003-H. PMID 1292654.
- Slapeta J, Moreira D, López-García P (2005). "The extent of protist diversity: insights from molecular ecology of freshwater eukaryotes". Proceedings of the Royal Society B: Biological Sciences. 272 (1576): 2073–81. doi:10.1098/rspb.2005.3195. PMC 1559898. PMID 16191619.
- Moreira D, López-García P (2002). "The molecular ecology of microbial eukaryotes unveils a hidden world" (PDF). Trends in Microbiology. 10 (1): 31–8. doi:10.1016/S0966-842X(01)02257-0. PMID 11755083.
- The Air You're Breathing? A Diatom Made That
- "More on Diatoms". University of California Museum of Paleontology. Archived from the original on 4 October 2012. Retrieved 27 June 2019.
- Devreotes P (1989). "Dictyostelium discoideum: a model system for cell-cell interactions in development". Science. 245 (4922): 1054–8. Bibcode:1989Sci...245.1054D. doi:10.1126/science.2672337. PMID 2672337.
- Matz, Mikhail V.; Tamara M. Frank; N. Justin Marshall; Edith A. Widder; Sonke Johnsen (9 December 2008). "Giant Deep-Sea Protist Produces Bilaterian-like Traces" (PDF). Current Biology. Elsevier Ltd. 18 (23): 1849–1854. doi:10.1016/j.cub.2008.10.028. PMID 19026540.
- Gooday, A. J.; Aranda da Silva, A.; Pawlowski, J. (1 December 2011). "Xenophyophores (Rhizaria, Foraminifera) from the Nazaré Canyon (Portuguese margin, NE Atlantic)". Deep-Sea Research Part II: Topical Studies in Oceanography. The Geology, Geochemistry, and Biology of Submarine Canyons West of Portugal. 58 (23–24): 2401–2419. Bibcode:2011DSRII..58.2401G. doi:10.1016/j.dsr2.2011.04.005.
- Neil A C, Reece J B, Simon E J (2004) Essential biology with physiology Pearson/Benjamin Cummings, Page 291. ISBN 9780805375039
- O'Malley MA, Simpson AG, Roger AJ (2012). "The other eukaryotes in light of evolutionary protistology". Biology & Philosophy. 28 (2): 299–330. doi:10.1007/s10539-012-9354-y.
- Adl SM, Simpson AG, Farmer MA, Andersen RA, Anderson OR, Barta JR, Bowser SS, Brugerolle G, Fensome RA, Fredericq S, James TY, Karpov S, Kugrens P, Krug J, Lane CE, Lewis LA, Lodge J, Lynn DH, Mann DG, McCourt RM, Mendoza L, Moestrup O, Mozley-Standridge SE, Nerad TA, Shearer CA, Smirnov AV, Spiegel FW, Taylor MF (2005). "The new higher level classification of eukaryotes with emphasis on the taxonomy of protists". The Journal of Eukaryotic Microbiology. 52 (5): 399–451. doi:10.1111/j.1550-7408.2005.00053.x. PMID 16248873.
- Margulis L, Chapman MJ (19 March 2009). Kingdoms and Domains: An Illustrated Guide to the Phyla of Life on Earth. Academic Press. ISBN 9780080920146.
- Janet Fang (6 April 2010). "Animals thrive without oxygen at sea bottom". Nature. 464 (7290): 825. doi:10.1038/464825b. PMID 20376121.
- "Briny deep basin may be home to animals thriving without oxygen". Science News. 23 September 2013.
- Hyde, K.D.; E.B.J. Jones; E. Leaño; S.B. Pointing; A.D. Poonyth; L.L.P. Vrijmoed (1998). "Role of fungi in marine ecosystems". Biodiversity and Conservation. 7 (9): 1147–1161. doi:10.1023/A:1008823515157.
- Kirk, P.M., Cannon, P.F., Minter, D.W. and Stalpers, J. "Dictionary of the Fungi". Edn 10. CABI, 2008
- Hyde, K.D.; E.B.J. Jones (1989). "Spore attachment in marine fungi". Botanica Marina. 32 (3): 205–218. doi:10.1515/botm.1989.32.3.205.
- Le Calvez T, Burgaud G, Mahé S, Barbier G, Vandenkoornhuyse P (October 2009). "Fungal diversity in deep-sea hydrothermal ecosystems". Applied and Environmental Microbiology. 75 (20): 6415–21. doi:10.1128/AEM.00653-09. PMC 2765129. PMID 19633124.
- San-Martín, A.; S. Orejanera; C. Gallardo; M. Silva; J. Becerra; R. Reinoso; M.C. Chamy; K. Vergara; J. Rovirosa (2008). "Steroids from the marine fungus Geotrichum sp". Journal of the Chilean Chemical Society. 53 (1): 1377–1378. doi:10.4067/S0717-97072008000100011.
- Jones, E.B.G., Hyde, K.D., & Pang, K.-L., eds. (2014). Freshwater fungi: and fungal-like organisms. Berlin/Boston: De Gruyter.
- Jones, E.B.G.; Pang, K.-L., eds. (2012). Marine Fungi, and Fungal-like Organisms. Marine and Freshwater Botany. Berlin, Boston: De Gruyter (published August 2012). doi:10.1515/9783110264067. ISBN 978-3-11-026406-7. Retrieved 3 September 2015.
- Wang, Xin; Singh, Purnima; Gao, Zheng; Zhang, Xiaobo; Johnson, Zackary I.; Wang, Guangyi (2014). "Distribution and Diversity of Planktonic Fungi in the West Pacific Warm Pool". PLOS ONE. 9 (7): e101523. Bibcode:2014PLoSO...9j1523W. doi:10.1371/journal.pone.0101523.s001. PMC 4081592. PMID 24992154.
- Wang, G.; Wang, X.; Liu, X.; Li, Q. (2012). "Diversity and biogeochemical function of planktonic fungi in the ocean". In Raghukumar, C. (ed.). Biology of marine fungi. Berlin, Heidelberg: Springer-Verlag. pp. 71–88. doi:10.1007/978-3-642-23342-5. ISBN 978-3-642-23341-8. Retrieved 3 September 2015.
- Damare, Samir; Raghukumar, Chandralata (11 November 2007). "Fungi and Macroaggregation in Deep-Sea Sediments". Microbial Ecology. 56 (1): 168–177. doi:10.1007/s00248-007-9334-y. ISSN 0095-3628. PMID 17994287.
- Kubanek, Julia; Jensen, Paul R.; Keifer, Paul A.; Sullards, M. Cameron; Collins, Dwight O.; Fenical, William (10 June 2003). "Seaweed resistance to microbial attack: A targeted chemical defense against marine fungi". Proceedings of the National Academy of Sciences. 100 (12): 6916–6921. Bibcode:2003PNAS..100.6916K. doi:10.1073/pnas.1131855100. ISSN 0027-8424. PMC 165804. PMID 12756301.
- Gao, Zheng; Johnson, Zackary I.; Wang, Guangyi (30 July 2009). "Molecular characterization of the spatial diversity and novel lineages of mycoplankton in Hawaiian coastal waters". The ISME Journal. 4 (1): 111–120. doi:10.1038/ismej.2009.87. ISSN 1751-7362. PMID 19641535.
- Panzer, Katrin; Yilmaz, Pelin; Weiß, Michael; Reich, Lothar; Richter, Michael; Wiese, Jutta; Schmaljohann, Rolf; Labes, Antje; Imhoff, Johannes F. (30 July 2015). "Identification of Habitat-Specific Biomes of Aquatic Fungal Communities Using a Comprehensive Nearly Full-Length 18S rRNA Dataset Enriched with Contextual Data". PLoS ONE. 10 (7): e0134377. Bibcode:2015PLoSO..1034377P. doi:10.1371/journal.pone.0134377. PMC 4520555. PMID 26226014.
- Gutierrez, Marcelo H; Pantoja, Silvio; Quinones, Renato a and Gonzalez, Rodrigo R. First record of flamentous fungi in the coastal upwelling ecosystem off central Chile. Gayana (Concepc.) [online]. 2010, vol.74, n.1, pp. 66-73. ISSN 0717-6538.
- Sridhar, K.R. (2009). "10. Aquatic fungi – Are they planktonic?". Plankton Dynamics of Indian Waters. Jaipur, India: Pratiksha Publications. pp. 133–148.
- Species of Higher Marine Fungi Archived 22 April 2013 at the Wayback Machine University of Mississippi. Retrieved 2012-02-05.
- Freshwater and marine lichen-forming fungi Retrieved 2012-02-06.
- "Lichens". National Park Service, US Department of the Interior, Government of the United States. 22 May 2016. Retrieved 4 April 2018.
- "The Earth Life Web, Growth and Development in Lichens". earthlife.net.
- Silliman B. R. & S. Y. Newell (2003). "Fungal farming in a snail". PNAS. 100 (26): 15643–15648. Bibcode:2003PNAS..10015643S. doi:10.1073/pnas.2535227100. PMC 307621. PMID 14657360.
- Yuan X, Xiao S (2005). "Lichen-Like Symbiosis 600 Million Years Ago". Science. 308 (5724): 1017–1020. Bibcode:2005Sci...308.1017Y. doi:10.1126/science.1111347. PMID 15890881.
- Jones, E. B. Gareth; Pang, Ka-Lai (31 August 2012). Marine Fungi: and Fungal-like Organisms. Walter de Gruyter. ISBN 9783110264067.
- Davidson, Michael W. (26 May 2005). "Animal Cell Structure". Molecular Expressions. Tallahassee, Fla.: Florida State University. Retrieved 3 September 2008.
- Vogel, Gretchen (20 September 2018). "This fossil is one of the world's earliest animals, according to fat molecules preserved for a half-billion years". Science. AAAS. Retrieved 21 September 2018.
- Bobrovskiy, Ilya (2018). "Ancient steroids establish the Ediacaran fossil Dickinsonia as one of the earliest animals". Science. 361 (6408): 1246–1249. Bibcode:2018Sci...361.1246B. doi:10.1126/science.aat7228. PMID 30237355.
- Retallack, G.J. (2007). "Growth, decay and burial compaction of Dickinsonia, an iconic Ediacaran fossil" (PDF). Alcheringa: An Australasian Journal of Palaeontology. 31 (3): 215–240. doi:10.1080/03115510701484705.
- Sperling, Erik; Vinther, Jakob; Pisani, Davide; Peterson, Kevin (2008). "A placozoan affinity for Dickinsonia and the evolution of Late Precambrian metazoan feeding modes" (PDF). In Cusack, M; Owen, A; Clark, N (eds.). Programme with Abstracts. Palaeontological Association Annual Meeting. 52. Glasgow, UK. p. 81.
- Gold, D. A.; Runnegar, B.; Gehling, J. G.; Jacobs, D. K. (2015). "Ancestral state reconstruction of ontogeny supports a bilaterian affinity for Dickinsonia". Evolution & Development. 17 (6): 315–397. doi:10.1111/ede.12168. PMID 26492825.
- Jun-Yuan Chen; Oliveri, Paola; Feng Gao; et al. (1 August 2002). "Precambrian Animal Life: Probable Developmental and Adult Cnidarian Forms from Southwest China" (PDF). Developmental Biology. 248 (1): 182–196. doi:10.1006/dbio.2002.0714. ISSN 0012-1606. PMID 12142030. Archived from the original (PDF) on 26 May 2013. Retrieved 4 February 2015.CS1 maint: ref=harv (link)
- Grazhdankin, Dima (June 2004). "Patterns of distribution in the Ediacaran biotas: facies versus biogeography and evolution". Paleobiology. 30 (2): 203–221. doi:10.1666/0094-8373(2004)030<0203:PODITE>2.0.CO;2. ISSN 0094-8373.CS1 maint: ref=harv (link)
- Seilacher, Adolf (August 1992). "Vendobionta and Psammocorallia: lost constructions of Precambrian evolution". Journal of the Geological Society. 149 (4): 607–613. Bibcode:1992JGSoc.149..607S. doi:10.1144/gsjgs.149.4.0607. ISSN 0016-7649. Retrieved 4 February 2015.CS1 maint: ref=harv (link)
- Martin, Mark W.; Grazhdankin, Dmitriy V.; Bowring, Samuel A.; et al. (5 May 2000). "Age of Neoproterozoic Bilaterian Body and Trace Fossils, White Sea, Russia: Implications for Metazoan Evolution". Science. 288 (5467): 841–845. Bibcode:2000Sci...288..841M. doi:10.1126/science.288.5467.841. ISSN 0036-8075. PMID 10797002.CS1 maint: ref=harv (link)
- Fedonkin, Mikhail A.; Waggoner, Benjamin M. (28 August 1997). "The late Precambrian fossil Kimberella is a mollusc-like bilaterian organism". Nature. 388 (6645): 868–871. Bibcode:1997Natur.388..868F. doi:10.1038/42242. ISSN 0028-0836.CS1 maint: ref=harv (link)
- Mooi, Rich; David, Bruno (December 1998). "Evolution Within a Bizarre Phylum: Homologies of the First Echinoderms" (PDF). American Zoologist. 38 (6): 965–974. doi:10.1093/icb/38.6.965. ISSN 1540-7063. Retrieved 5 February 2015.CS1 maint: ref=harv (link)
- McMenamin, Mark A. S. (September 2003). Spriggina is a trilobitoid ecdysozoan. Geoscience Horizons Seattle 2003. Abstracts with Programs. 35. Boulder, Colo.: Geological Society of America. p. 105. OCLC 249088612. Archived from the original on 12 April 2016. Retrieved 24 November 2007.CS1 maint: ref=harv (link) Paper No. 40-2 presented at the Geological Society of America's 2003 Seattle Annual Meeting (2–5 November 2003) on 2 November 2003, at the Washington State Convention Center.
- Jih-Pai Lin; Gon, Samuel M., III; Gehling, James G.; et al. (2006). "A Parvancorina-like arthropod from the Cambrian of South China". Historical Biology: An International Journal of Paleobiology. 18 (1): 33–45. doi:10.1080/08912960500508689. ISSN 1029-2381.
- Butterfield, Nicholas J. (December 2006). "Hooking some stem-group 'worms': fossil lophotrochozoans in the Burgess Shale". BioEssays. 28 (12): 1161–1166. doi:10.1002/bies.20507. ISSN 0265-9247. PMID 17120226.CS1 maint: ref=harv (link)
- Bengtson 2004, pp. 67–78
- Valentine, James W. (2004). On the Origin of Phyla. Chicago: University Of Chicago Press. p. 7. ISBN 978-0-226-84548-7.
Classifications of organisms in hierarchical systems were in use by the seventeenth and eighteenth centuries. Usually organisms were grouped according to their morphological similarities as perceived by those early workers, and those groups were then grouped according to their similarities, and so on, to form a hierarchy.
CS1 maint: ref=harv (link) - Valentine, James W (18 June 2004). On the Origin of Phyla. ISBN 9780226845487.
- WoRMS Editorial Board. World Register of Marine Species. Available online: http://www.marinespecies.org at VLIZ. Accessed: 18 October 2019.
- Novak, B.J.; Fraser, D.; Maloney, T.H. (2020). "Transforming ocean conservation: applying the genetic rescue toolkit". Genes. 11 (2): 209. doi:10.3390/genes11020209.
- Gould, Stephen Jay (1990) Wonderful Life: The Burgess Shale and the Nature of History W. W. Norton. ISBN 9780393307009.
- Erwin, Douglas; Valentine, James; Jablonski, David (1997). "Recent fossil finds and new insights into animal development are providing fresh perspectives on the riddle of the explosion of animals during the Early Cambrian". American Scientist (March–April).
- Budd, G.E.; Jensen, S. (May 2000). "A critical reappraisal of the fossil record of the bilaterian phyla". Biological Reviews. 75 (2): 253–295. doi:10.1111/j.1469-185X.1999.tb00046.x. PMID 10881389.
- Gould 1989
- Budd, Graham E. (February 2003). "The Cambrian Fossil Record and the Origin of the Phyla" (PDF). Integrative and Comparative Biology. 43 (1): 157–165. doi:10.1093/icb/43.1.157. ISSN 1557-7023. PMID 21680420. Retrieved 6 February 2015.CS1 maint: ref=harv (link)
- Budd, Graham E. (March 1996). "The morphology of Opabinia regalis and the reconstruction of the arthropod stem-group". Lethaia. 29 (1): 1–14. doi:10.1111/j.1502-3931.1996.tb01831.x. ISSN 0024-1164.CS1 maint: ref=harv (link)
- Marshall, Charles R. (May 2006). "Explaining the Cambrian 'Explosion' of Animals". Annual Review of Earth and Planetary Sciences. 34: 355–384. Bibcode:2006AREPS..34..355M. doi:10.1146/annurev.earth.33.031504.103001. ISSN 1545-4495.CS1 maint: ref=harv (link)
- King, N.; Rokas, A. (2017). "Embracing uncertainty in reconstructing early animal evolution". Current Biology. 27 (19): R1081–R1088. doi:10.1016/j.cub.2017.08.054.
- Feuda, R.; Dohrmann, M.; Pett, W.; Philippe, H.; Rota-Stabelli, O.; Lartillot, N.; Wörheide, G.; Pisani, D. (2017). "Improved modeling of compositional heterogeneity supports sponges as sister to all other animals". Current Biology. 27 (24): 3864–3870. doi:10.1016/j.cub.2017.11.008. PMID 29199080.
- Nielsen, Claus (2019). ""Early animal evolution: a morphologist's view", review article". Royal Society Open Science. 6: 7. doi:10.1098/rsos.190638.
- Porifera (n.) Online Etymology Dictionary. Retrieved 18 August 2016.
- Petralia, R.S.; Mattson, M.P.; Yao, P.J. (2014). "Aging and longevity in the simplest animals and the quest for immortality". Ageing Research Reviews. 16: 66–82. doi:10.1016/j.arr.2014.05.003. PMC 4133289. PMID 24910306.
- Jochum, K.P.; Wang, X.; Vennemann, T.W.; Sinha, B.; Müller, W.E. (2012). "Siliceous deep-sea sponge Monorhaphis chuni: A potential paleoclimate archive in ancient animals". Chemical Geology. 300: 143–151. Bibcode:2012ChGeo.300..143J. doi:10.1016/j.chemgeo.2012.01.009.
- Vacelet & Duport 2004, pp. 179–190.
- "Spongia Linnaeus, 1759". World Register of Marine Species. Retrieved 18 July 2012.
- Rowland, S. M. & Stephens, T. (2001). "Archaeocyatha: A history of phylogenetic interpretation". Journal of Paleontology. 75 (6): 1065–1078. doi:10.1666/0022-3360(2001)075<1065:AAHOPI>2.0.CO;2. JSTOR 1307076. Archived from the original on 6 December 2008. Retrieved 18 August 2016.
- Sperling, E. A.; Pisani, D.; Peterson, K. J. (1 January 2007). "Poriferan paraphyly and its implications for Precambrian palaeobiology" (PDF). Geological Society, London, Special Publications. 286 (1): 355–368. Bibcode:2007GSLSP.286..355S. doi:10.1144/SP286.25. Archived from the original (PDF) on 20 December 2009. Retrieved 22 August 2012.
- Ruppert, E.E.; Fox, R.S. & Barnes, R.D. (2004). Invertebrate Zoology (7 ed.). Brooks / Cole. pp. 182–195. ISBN 978-0-03-025982-1.
- Mills, C.E. "Ctenophores – some notes from an expert". Retrieved 5 February 2009.
- Brusca R. C. and Brusca G. J. (2003) Invertebrates, Second Edition, Sinauer Associates. ISBN 9780878930975.
- Michael Le Page (March 2019). "Animal with an anus that comes and goes could reveal how ours evolved". New Scientist.
- Martindale, Mark; Finnerty, J.R.; Henry, J.Q. (September 2002). "The Radiata and the evolutionary origins of the bilaterian body plan". Molecular Phylogenetics and Evolution. 24 (3): 358–365. doi:10.1016/s1055-7903(02)00208-7.
- Placozoa at the US National Library of Medicine Medical Subject Headings (MeSH)
- Rüdiger Wehner & Walter Gehring (June 2007). Zoologie (in German) (24th ed.). Stuttgart: Thieme. p. 696.
- F. E. Schulze "Trichoplax adhaerens n. g., n. s.", Zoologischer Anzeiger (Elsevier, Amsterdam and Jena) 6 (1883), p. 92.
- Eitel, Michael; Francis, Warren; Osigus, Hans-Jürgen; Krebs, Stefan; Vargas, Sergio; Blum, Helmut; Williams, Gray Argust; Schierwater, Bernd; Wörheide, Gert (13 October 2017). "A taxogenomics approach uncovers a new genus in the phylum Placozoa". bioRxiv: 202119. doi:10.1101/202119.
- Schierwater, Bernd; Kamm, Kai; Herzog, Rebecca; Rolfes, Sarah; Osigus, Hans-Jürgen (4 March 2019). "Polyplacotoma mediterranea is a new ramified placozoan species". Current Biology. 29 (5): R148–R149. doi:10.1016/j.cub.2019.01.068. ISSN 0960-9822. PMID 30836080.
- Trichoplax adhaerens, WoRMS, 2009.
- Smith, Carolyn L.; Varoqueaux, Frédérique; Kittelmann, Maike; Azzam, Rita N.; Cooper, Benjamin; Winters, Christine A.; Eitel, Michael; Fasshauer, Dirk; Reese, Thomas S. (2014). "Novel Cell Types, Neurosecretory Cells, and Body Plan of the Early-Diverging Metazoan Trichoplax adhaerens". Current Biology. 24 (14): 1565–1572. doi:10.1016/j.cub.2014.05.046. ISSN 0960-9822. PMC 4128346. PMID 24954051.
- Barnes, Robert D. (1982). Invertebrate Zoology. Philadelphia: Holt-Saunders International. pp. 84–85. ISBN 978-0-03-056747-6.
- Zhang, Z.-Q. (2011). "Animal biodiversity: An introduction to higher-level classification and taxonomic richness" (PDF). Zootaxa. 3148: 7–12. doi:10.11646/zootaxa.3148.1.3.
- "Nematostella vectensis v1.0". Genome Portal. University of California. Retrieved 19 January 2014.
- "Nematostella". Nematostella.org. Archived from the original on 8 May 2006. Retrieved 18 January 2014.
- Genikhovich, G. & U. Technau (2009). "The Starlet sea anemone Nematostella vectensis: An anthozoan model organism for studies in comparative genomics and functional evolutionary developmental biology". Cold Spring Harbor Protocols. 2009 (9): pdb.emo129. doi:10.1101/pdb.emo129. PMID 20147257.
- "Where Does Our Head Come From? Brainless Sea Anemone Sheds New Light on the Evolutionary Origin of the Head". Science Daily. 12 February 2013. Retrieved 18 January 2014.
- Sinigaglia, C.; et al. (2013). "The bilaterian head patterning gene six3/6 controls aboral domain development in a cnidarian". PLoS Biology. 11 (2): e1001488. doi:10.1371/journal.pbio.1001488. PMC 3586664. PMID 23483856.
- "Red Paper Lantern Jellyfish". Real Monstrosities. Retrieved 25 October 2015.
- "Blue Buttons in Florida."
- Karleskint G, Richard Turner R and, James Small J (2012) Introduction to Marine Biology Cengage Learning, edition 4, page 445. ISBN 9781133364467.
- Bavestrello, Giorgio; Christian Sommer; Michele Sarà (1992). "Bi-directional conversion in Turritopsis nutricula (Hydrozoa)". Scientia Marina. 56 (2–3): 137–140.
- Piraino, Stefano; F. Boero; B. Aeschbach; V. Schmid (1996). "Reversing the life cycle: medusae transforming into polyps and cell transdifferentiation in Turritopsis nutricula (Cnidaria, Hydrozoa)". Biological Bulletin. 190 (3): 302–312. doi:10.2307/1543022. JSTOR 1543022. PMID 29227703.
- Fenner PJ, Williamson JA (1996). "Worldwide deaths and severe envenomation from jellyfish stings". The Medical Journal of Australia. 165 (11–12): 658–61. doi:10.5694/j.1326-5377.1996.tb138679.x. PMID 8985452.
- Cannon, Johanna Taylor; Vellutini, Bruno Cossermelli; Smith, Julian; Ronquist, Fredrik; Jondelius, Ulf; Hejnol, Andreas (2016). "Xenacoelomorpha is the sister group to Nephrozoa". Nature. 530 (7588): 89–93. Bibcode:2016Natur.530...89C. doi:10.1038/nature16520. PMID 26842059.
- Minelli, Alessandro (2009). Perspectives in Animal Phylogeny and Evolution. Oxford University Press. p. 53. ISBN 978-0-19-856620-5.
- Brusca, Richard C. (2016). Introduction to the Bilateria and the Phylum Xenacoelomorpha | Triploblasty and Bilateral Symmetry Provide New Avenues for Animal Radiation (PDF). Invertebrates. Sinauer Associates. pp. 345–372. ISBN 978-1605353753.
- Finnerty, John R. (November 2005). "Did internal transport, rather than directed locomotion, favor the evolution of bilateral symmetry in animals?" (PDF). BioEssays. 27 (11): 1174–1180. doi:10.1002/bies.20299. PMID 16237677. Archived from the original (PDF) on 10 August 2014. Retrieved 27 August 2019.
- Quillin, K. J. (May 1998). "Ontogenetic scaling of hydrostatic skeletons: geometric, static stress and dynamic stress scaling of the earthworm lumbricus terrestris". The Journal of Experimental Biology. 201 (12): 1871–83. PMID 9600869.
- This primeval worm may be the ancestor of all animals Live Science, 26 March 2020.
- Cannon, J.T.; Vellutini, B.C.; Smith, J.; Ronquist, F.; Jondelius, U.; Hejnol, A. (2016). "Xenacoelomorpha is the sister group to Nephrozoa". Nature. 530: 89–93. doi:10.1038/nature16520. PMID 26842059.
- Wade, Nicholas (30 January 2017). "This Prehistoric Human Ancestor Was All Mouth". The New York Times. Retrieved 31 January 2017.
- Han, Jian; Morris, Simon Conway; Ou, Qiang; Shu, Degan; Huang, Hai (2017). "Meiofaunal deuterostomes from the basal Cambrian of Shaanxi (China)". Nature. 542 (7640): 228–231. Bibcode:2017Natur.542..228H. doi:10.1038/nature21072. ISSN 0028-0836. PMID 28135722.
- "Cornwall – Nature – Superstar Worm". BBC.
- Mark Carwardine (1995) The Guinness Book of Animal Records. Guinness Publishing. p. 232.
- "The Persistent Parasites". Time. 8 April 1957.
- Hargis, William J. (1985). "Parasitology and pathology of marine organisms of the world ocean". National Oceanic and Atmospheric Administration. Cite journal requires
|journal=(help) - http://plpnemweb.ucdavis.edu/nemaplex/General/animpara.htm
- Lynne S. Garcia (1999). "Classification of Human Parasites, Vectors, and Similar Organisms" (PDF). Clin Infect Dis. 29 (4): 734–6. doi:10.1086/520425. PMID 10589879.
- Hodda, M (2011). "Phylum Nematoda Cobb, 1932. In: Zhang, Z.-Q. Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness". Zootaxa. 3148: 63–95. doi:10.11646/zootaxa.3148.1.11.
- Zhang, Z (2013). "Animal biodiversity: An update of classification and diversity in 2013. In: Zhang, Z.-Q. (Ed.) Animal Biodiversity: An Outline of Higher-level Classification and Survey of Taxonomic Richness (Addenda 2013)". Zootaxa. 3703 (1): 5–11. doi:10.11646/zootaxa.3703.1.3.
- Lambshead PJD (1993). "Recent developments in marine benthic biodiversity research". Oceanis. 19 (6): 5–24.
- Borgonie G, García-Moyano A, Litthauer D, Bert W, Bester A, van Heerden E, Möller C, Erasmus M, Onstott TC (June 2011). "Nematoda from the terrestrial deep subsurface of South Africa". Nature. 474 (7349): 79–82. Bibcode:2011Natur.474...79B. doi:10.1038/nature09974. hdl:1854/LU-1269676. PMID 21637257.
- Danovaro R, Gambi C, Dell'Anno A, Corinaldesi C, Fraschetti S, Vanreusel A, Vincx M, Gooday AJ (January 2008). "Exponential decline of deep-sea ecosystem functioning linked to benthic biodiversity loss". Current Biology. 18 (1): 1–8. doi:10.1016/j.cub.2007.11.056. PMID 18164201. Lay summary – EurekAlert!.
- Platt HM (1994). "foreword". In Lorenzen S, Lorenzen SA (eds.). The phylogenetic systematics of freeliving nematodes. London: The Ray Society. ISBN 978-0-903874-22-9.
- Barnes, R.S.K.; Calow, P.; Olive, P.J.W.; Golding, D.W. & Spicer, J.I. (2001). The Invertebrates, A Synthesis (3rd ed.). UK: Blackwell Science.
- Tyrian Purple Green Lion, 28 February 2014.
- Chapman, A.D. (2009). Numbers of Living Species in Australia and the World, 2nd edition. Australian Biological Resources Study, Canberra. Retrieved 12 January 2010. ISBN 978-0-642-56860-1 (printed); ISBN 978-0-642-56861-8 (online).
- Hancock, Rebecca (2008). "Recognising research on molluscs". Australian Museum. Archived from the original on 30 May 2009. Retrieved 9 March 2009.
- Ponder, W.F.; Lindberg, D.R., eds. (2008). Phylogeny and Evolution of the Mollusca. Berkeley: University of California Press. p. 481. ISBN 978-0-520-25092-5.
- Munro, D.; Blier, P.U. (2012). "The extreme longevity of Arctica islandica is associated with increased peroxidation resistance in mitochondrial membranes". Aging Cell. 11 (5): 845–55. doi:10.1111/j.1474-9726.2012.00847.x. PMID 22708840.
- "Welcome to CephBase". CephBase. Retrieved 29 January 2016.
- Wilbur, Karl M.; Clarke, M.R.; Trueman, E.R., eds. (1985), The Mollusca, 12. Paleontology and neontology of Cephalopods, New York: Academic Press, ISBN 0-12-728702-7
- "Are there any freshwater cephalopods?". 16 January 2013.
- Ewen Callaway (2 June 2008). "Simple-Minded Nautilus Shows Flash of Memory". New Scientist. Retrieved 7 March 2012.
- Kathryn Phillips (15 June 2008). "Living Fossil Memories". Journal of Experimental Biology. 211 (12): iii. doi:10.1242/jeb.020370.
- Robyn Crook & Jennifer Basil (2008). "A biphasic memory curve in the chambered nautilus, Nautilus pompilius L. (Cephalopoda: Nautiloidea)". Journal of Experimental Biology. 211 (12): 1992–1998. doi:10.1242/jeb.018531. PMID 18515730.
- Black, Richard (26 April 2008). "Colossal squid out of the freezer". BBC News.
- Ruppert, Edward E.; Fox, Richard, S.; Barnes, Robert D. (2004). Invertebrate Zoology (7th ed.). Cengage Learning. ISBN 978-81-315-0104-7.
- Hayward, PJ (1996). Handbook of the Marine Fauna of North-West Europe. Oxford University Press. ISBN 978-0-19-854055-7.
- Ruppert, Fox & Barnes (2004), pp. 518–522
- Wilson, Heather M.; Anderson, Lyall I. (January 2004). "Morphology and taxonomy of Paleozoic millipedes (Diplopoda: Chilognatha: Archipolypoda) from Scotland". Journal of Paleontology. 78 (1): 169–184. Bibcode:1974JPal...48..524M. doi:10.1666/0022-3360(2004)078<0169:MATOPM>2.0.CO;2.CS1 maint: ref=harv (link)
- Stephanie E. Suarez; Michael E. Brookfield; Elizabeth J. Catlos; Daniel F. Stöckli (2017). "A U-Pb zircon age constraint on the oldest-recorded air-breathing land animal". PLoS ONE. 12 (6): e0179262. Bibcode:2017PLoSO..1279262S. doi:10.1371/journal.pone.0179262. PMC 5489152. PMID 28658320.
- Campbell, L.I.; Rota-Stabelli, O.; Edgecombe, G.D.; Marchioro, T.; Longhorn, S.J.; Telford, M.J.; Philippe, H.; Rebecchi, L.; Peterson, K.J.; Pisani, D. (2011). "MicroRNAs and phylogenomics resolve the relationships of Tardigrada and suggest that velvet worms are the sister group of Arthropoda". Proceedings of the National Academy of Sciences. 108 (38): 15920–15924. Bibcode:2011PNAS..10815920C. doi:10.1073/pnas.1105499108. PMC 3179045. PMID 21896763.
- Smith, F.W.; Goldstein, B. (2017). "Segmentation in Tardigrada and diversification of segmental patterns in Panarthropoda". Arthropod Structure & Development. 46 (3): 328–340. doi:10.1016/j.asd.2016.10.005. PMID 27725256.
- Budd, G. E. (2001). "Why are arthropods segmented?". Evolution and Development. 3 (5): 332–42. doi:10.1046/j.1525-142X.2001.01041.x. PMID 11710765.
- "Archived copy". Archived from the original on 26 January 2011. Retrieved 10 March 2011.CS1 maint: archived copy as title (link)
- Braddy, Simon J.; Poschmann, Markus; Tetlie, O. Erik (2007). "Giant claw reveals the largest ever arthropod". Biology Letters. 4 (1): 106–109. doi:10.1098/rsbl.2007.0491. PMC 2412931. PMID 18029297.
- Cressey, Daniel (21 November 2007). "Giant sea scorpion discovered". Nature. doi:10.1038/news.2007.272. Retrieved 10 June 2013.
- "An ugly giant crab of Japan". Popular Science. 96 (6): 42. 1920.
- D. R. Currie & T. M. Ward (2009). South Australian Giant Crab (Pseudocarcinus gigas) Fishery (PDF). South Australian Research and Development Institute. Fishery Assessment Report for PIRSA. Retrieved 9 December 2013.
- Patrick Kilday (28 September 2005). "Mantis shrimp boasts most advanced eyes". The Daily Californian.
- S. N. Patek & R. L. Caldwell (2005). "Extreme impact and cavitation forces of a biological hammer: strike forces of the peacock mantis shrimp". Journal of Experimental Biology. 208 (19): 3655–3664. doi:10.1242/jeb.01831. PMID 16169943.
- Ghosh, Pallab (30 January 2017). "Scientists find 'oldest human ancestor'". BBC. Retrieved 30 January 2017.
- "Animal Diversity Web - Echinodermata". University of Michigan Museum of Zoology. Retrieved 26 August 2012.
- "Sea Lily". Science Encyclopedia. Retrieved 5 September 2014.
- Fox, Richard. "Asterias forbesi". Invertebrate Anatomy OnLine. Lander University. Retrieved 14 June 2014.
- Holsinger, K. (2005). Keystone species. Retrieved 10 May 2010, from "Archived copy". Archived from the original on 30 June 2010. Retrieved 12 May 2010.CS1 maint: archived copy as title (link)
- Simakov, O.; Kawashima, T.; Marlétaz, F.; Jenkins, J.; Koyanagi, R.; Mitros, T.; Hisata, K.; Bredeson, J.; Shoguchi, E.; Gyoja, F.; Yue, J.X. (2015). "Hemichordate genomes and deuterostome origins". Nature. 527 (7579): 459–65. Bibcode:2015Natur.527..459S. doi:10.1038/nature16150. PMC 4729200. PMID 26580012.
- How humans got a pharynx from this 'ugly beast', Futurity, 23 November 2015.
- The secret to an Oesia life: Prehistoric worm built tube-like 'houses' on sea floor
- Barnes, Robert D. (1982). Invertebrate Zoology. Philadelphia, PA: Holt-Saunders International. pp. 1018–1026. ISBN 978-0-03-056747-6.
- Secondary organizers of the early brain and the location of the meso-diencephalic dopaminergic precursor cells Retrieved March 10, 2014
- Rob Mitchum (15 March 2012). "The Secret Origin of the Vertebrate Brain". Nature. ScienceLife. 483 (7389): 289–94. doi:10.1038/nature10838. PMC 3719855. PMID 22422262. Retrieved 18 February 2014.
- Gewin, V (2005). "Functional genomics thickens the biological plot". PLoS Biology. 3 (6): e219. doi:10.1371/journal.pbio.0030219. PMC 1149496. PMID 15941356.
- Lancelet (amphioxus) genome and the origin of vertebrates Ars Technica, 19 June 2008.
- Lemaire, P (2011). "Evolutionary crossroads in developmental biology: the tunicates". Development. 138 (11): 2143–2152. doi:10.1242/dev.048975. PMID 21558365.
- "FishBase: A Global Information System on Fishes". FishBase. Retrieved 17 January 2017.
- "How Many Fish In The Sea? Census Of Marine Life Launches First Report". Science Daily. Retrieved 17 January 2017.
- Docker, Margaret F. (2006). "Bill Beamish's Contributions to Lamprey Research and Recent Advances in the Field". Guelph Ichthyology Reviews. 7. doi:10.1111/j.1095-8649.2006.00968.x (inactive 11 February 2020).
- Hardisty, M. W.; Potter, I. C. (1971). Hardisty, M. W.; Potter, I. C. (eds.). The Biology of Lampreys (1st ed.). Academic Press. ISBN 978-0-123-24801-5.
- Gill, Howard S.; Renaud, Claude B.; Chapleau, François; Mayden, Richard L.; Potter, Ian C.; Douglas, M. E. (2003). "Phylogeny of Living Parasitic Lampreys (Petromyzontiformes) Based on Morphological Data". Copeia. 2003 (4): 687–703. doi:10.1643/IA02-085.1.
- "Myxini". University of California Museum of Paleontology. Retrieved 17 January 2017.
- Greena, Stephen A.; Bronner, Marianne E. (2014). "The Lamprey: A jawless vertebrate model system for examining origin of the neural crest and other vertebrate traits". Differentiation. 87 (1–2): 44–51. doi:10.1016/j.diff.2014.02.001. PMC 3995830. PMID 24560767.
- Stock, D.; Whitt, G.S. (7 August 1992). "Evidence from 18S ribosomal RNA sequences that lampreys and hagfish form a natural group". Science. 257 (5071): 787–9. Bibcode:1992Sci...257..787S. doi:10.1126/science.1496398. PMID 1496398.
- Nicholls, H. (10 September 2009). "Mouth to Mouth". Nature. 461 (7261): 164–166. doi:10.1038/461164a. PMID 19741680.
- Kimmel, C. B.; Miller, C. T.; Keynes, R. J. (2001). "Neural crest patterning and the evolution of the jaw". Journal of Anatomy. 199 (1&2): 105–119. doi:10.1017/S0021878201008068. PMC 1594948. PMID 11523812.
- Gai, Z.; Zhu, M. (2012). "The origin of the vertebrate jaw: Intersection between developmental biology-based model and fossil evidence". Chinese Science Bulletin. 57 (30): 3819–3828. Bibcode:2012ChSBu..57.3819G. doi:10.1007/s11434-012-5372-z.
- Maisey, J. G. (2000). Discovering Fossil Fishes. Westview Press. pp. 1–223. ISBN 978-0-8133-3807-1.
- Wroe, S.; Huber, D. R.; Lowry, M.; McHenry, C.; Moreno, K.; Clausen, P.; Ferrara, T. L.; Cunningham, E.; Dean, M. N.; Summers, A. P. (2008). "Three-dimensional computer analysis of white shark jaw mechanics: how hard can a great white bite?" (PDF). Journal of Zoology. 276 (4): 336–342. doi:10.1111/j.1469-7998.2008.00494.x.
- Pimiento, Catalina; Dana J. Ehret; Bruce J. MacFadden; Gordon Hubbell (10 May 2010). Stepanova, Anna (ed.). "Ancient Nursery Area for the Extinct Giant Shark Megalodon from the Miocene of Panama". PLoS ONE. 5 (5): e10552. Bibcode:2010PLoSO...510552P. doi:10.1371/journal.pone.0010552. PMC 2866656. PMID 20479893.
- Lambert, Olivier; Bianucci, Giovanni; Post, Klaas; de Muizon, Christian; Salas-Gismondi, Rodolfo; Urbina, Mario; Reumer, Jelle (1 July 2010). "The giant bite of a new raptorial sperm whale from the Miocene epoch of Peru". Nature. 466 (7302): 105–108. Bibcode:2010Natur.466..105L. doi:10.1038/nature09067. PMID 20596020.
- Nielsen, Julius; Hedeholm, Rasmus B.; Heinemeier, Jan; Bushnell, Peter G.; Christiansen, Jørgen S.; Olsen, Jesper; Ramsey, Christopher Bronk; Brill, Richard W.; Simon, Malene; Steffensen, Kirstine F.; Steffensen, John F. (2016). "Eye lens radiocarbon reveals centuries of longevity in the Greenland shark (Somniosus microcephalus)". Science. 353 (6300): 702–4. Bibcode:2016Sci...353..702N. doi:10.1126/science.aaf1703. PMID 27516602. Lay summary – Sci News (12 August 2016).
- Marshall, A.; Bennett, M.B.; Kodja, G.; Hinojosa-Alvarez, S.; Galvan-Magana, F.; Harding, M.; Stevens, G. & Kashiwagi, T. (2011). "Manta birostris". IUCN Red List of Threatened Species. 2011: e.T198921A9108067. doi:10.2305/IUCN.UK.2011-2.RLTS.T198921A9108067.en.
- Black, Richard (11 June 2007). "Sawfish protection acquires teeth". BBC News.
- Thomas J. Near; et al. (2012). "Resolution of ray-finned fish phylogeny and timing of diversification". PNAS. 109 (34): 13698–13703. Bibcode:2012PNAS..10913698N. doi:10.1073/pnas.1206625109. PMC 3427055. PMID 22869754.
- Zhu, M; Zhao, W; Jia, L; Lu, J; Qiao, T; Qu, Q (2009). "The oldest articulated osteichthyan reveals mosaic gnathostome characters". Nature. 458 (7237): 469–474. Bibcode:2009Natur.458..469Z. doi:10.1038/nature07855. PMID 19325627.
- Clack, J. A. (2002) Gaining Ground. Indiana University
- "Chondrosteans: Sturgeon Relatives". paleos.com. Archived from the original on 25 December 2010.
- López-Arbarello, A (2012). "Phylogenetic Interrelationships of Ginglymodian Fishes (Actinopterygii: Neopterygii)". PLoS ONE. 7 (7): e39370. Bibcode:2012PLoSO...739370L. doi:10.1371/journal.pone.0039370. PMC 3394768. PMID 22808031.
- Berra, Tim M. (2008). Freshwater Fish Distribution. University of Chicago Press. p. 55. ISBN 978-0-226-04443-9.
- Lackmann, Alec R.; Andrews, Allen H.; Butler, Malcolm G.; Bielak-Lackmann, Ewelina S.; Clark, Mark E. (23 May 2019). "Bigmouth Buffalo Ictiobus cyprinellus sets freshwater teleost record as improved age analysis reveals centenarian longevity". Communications Biology. 2 (1): 197. doi:10.1038/s42003-019-0452-0. ISSN 2399-3642. PMC 6533251. PMID 31149641.
- Benton, Michael (2005). "The Evolution of Fishes After the Devonian". Vertebrate Palaeontology (3rd ed.). John Wiley & Sons. pp. 175–84. ISBN 978-1-4051-4449-0.
- Bone, Q.; Moore, R. (2008). Biology of Fishes. Garland Science. p. 29. ISBN 978-0-415-37562-7.
- Dorit, R. L.; Walker, W. F.; Barnes, R. D. (1991). Zoology. Saunders College Publishing. pp. 67–69. ISBN 978-0-03-030504-7.
- "Scientists Describe the World's Smallest, Lightest Fish". Scripps Institution of Oceanography. 20 July 2004. Retrieved 9 April 2016.
- Roach, John (13 May 2003). "World's Heaviest Bony Fish Discovered?". National Geographic News. Retrieved 9 January 2016.
- Narkiewicz, Katarzyna; Narkiewicz, Marek (January 2015). "The age of the oldest tetrapod tracks from Zachełmie, Poland". Lethaia. 48 (1): 10–12. doi:10.1111/let.12083. ISSN 0024-1164.
- Long JA, Gordon MS (September–October 2004). "The greatest step in vertebrate history: a paleobiological review of the fish-tetrapod transition". Physiol. Biochem. Zool. 77 (5): 700–19. doi:10.1086/425183. PMID 15547790.CS1 maint: ref=harv (link) as PDF
- Shubin, N. (2008). Your Inner Fish: A Journey Into the 3.5-Billion-Year History of the Human Body. New York: Pantheon Books. ISBN 978-0-375-42447-2.
- Laurin, M. (2010). How Vertebrates Left the Water. Berkeley, California, USA.: University of California Press. ISBN 978-0-520-26647-6.
- Canoville, Aurore; Laurin, Michel (2010). "Evolution of humeral microanatomy and lifestyle in amniotes, and some comments on paleobiological inferences". Biological Journal of the Linnean Society. 100 (2): 384–406. doi:10.1111/j.1095-8312.2010.01431.x.CS1 maint: ref=harv (link)
- Laurin, Michel; Canoville, Aurore; Quilhac, Alexandra (2009). "Use of paleontological and molecular data in supertrees for comparative studies: the example of lissamphibian femoral microanatomy". Journal of Anatomy. 215 (2): 110–123. doi:10.1111/j.1469-7580.2009.01104.x. PMC 2740958. PMID 19508493.CS1 maint: ref=harv (link)
- Hopkins Gareth R.; Brodie Edmund D. Jr (2015). "Occurrence of Amphibians in Saline Habitats: A Review and Evolutionary Perspective". Herpetological Monographs. 29 (1): 1–27. doi:10.1655/HERPMONOGRAPHS-D-14-00006.
- Natchev, Nikolay; Tzankov, Nikolay; Geme, Richard (2011). "Green frog invasion in the Black Sea: habitat ecology of the Pelophylax esculentus complex (Anura, Amphibia) population in the region of Shablenska Тuzla lagoon in Bulgaria" (PDF). Herpetology Notes. 4: 347–351.
- Sander, P. Martin (2012). "Reproduction in early amniotes". Science. 337 (6096): 806–808. Bibcode:2012Sci...337..806S. doi:10.1126/science.1224301. PMID 22904001.
- Modesto, S.P.; Anderson, J.S. (2004). "The phylogenetic definition of Reptilia". Systematic Biology. 53 (5): 815–821. doi:10.1080/10635150490503026. PMID 15545258.

- Gauthier, J.A.; Kluge, A.G.; Rowe, T. (1988). "The early evolution of the Amniota". In Benton, M.J. (ed.). The Phylogeny and Classification of the Tetrapods. 1. Oxford: Clarendon Press. pp. 103–155. ISBN 978-0-19-857705-8.
- Laurin, M.; Reisz, R. R. (1995). "A reevaluation of early amniote phylogeny" (PDF). Zoological Journal of the Linnean Society. 113 (2): 165–223. doi:10.1111/j.1096-3642.1995.tb00932.x. Archived from the original (PDF) on 8 June 2019. Retrieved 14 August 2016.

- Modesto, S.P. (1999). "Observations of the structure of the Early Permian reptile Stereosternum tumidum Cope". Palaeontologia Africana. 35: 7–19.
- Rasmussen, Arne Redsted; Murphy, John C.; Ompi, Medy; Gibbons, J. Whitfield; Uetz, Peter (8 November 2011). "Marine Reptiles". PLoS ONE. 6 (11): e27373. Bibcode:2011PLoSO...627373R. doi:10.1371/journal.pone.0027373. PMC 3210815. PMID 22087300.
- Stidworthy J. 1974. Snakes of the World. Grosset & Dunlap Inc. 160 pp. ISBN 0-448-11856-4.
- Sea snakes at Food and Agriculture Organization of the United Nations. Accessed 7 August 2007.
- Rasmussen, A.R.; Murphy, J.C.; Ompi, M.; Gibbons, J.W.; Uetz, P. (2011). "Marine reptiles". PLOS ONE. 6 (11): e27373. Bibcode:2011PLoSO...627373R. doi:10.1371/journal.pone.0027373. PMC 3210815. PMID 22087300.
- Martill D.M. (1993). "Soupy Substrates: A Medium for the Exceptional Preservation of Ichthyosaurs of the Posidonia Shale (Lower Jurassic) of Germany". Kaupia - Darmstädter Beiträge zur Naturgeschichte, 2 : 77-97.
- Gould, Stephen Jay (1993) "Bent Out of Shape" in Eight Little Piggies: Reflections in Natural History. Norton, 179–94. ISBN 9780393311396.
- "Sardine Run Shark Feeding Frenzy Phenomenon in Africa". Archived from the original on 2 December 2008.
- "The Society for Marine Mammalogy's Taxonomy Committee List of Species and subspecies". Society for Marine Mammalogy. October 2015. Archived from the original on 6 January 2015. Retrieved 23 November 2015.
- Romer A. S. and Parsons T. S. (1986) The Vertebrate Body, page 96, Sanders College Publishing. ISBN 0030584469.
- "Blue whale". World Wide Fund For Nature. Retrieved 15 August 2016.
- Marino, Lori (2004). "Cetacean Brain Evolution: Multiplication Generates Complexity" (PDF). International Society for Comparative Psychology (17): 1–16. Archived from the original (PDF) on 16 September 2018. Retrieved 15 August 2016.
- Campbell, Neil A.; Reece, Jane B.; Urry, Lisa Andrea; Cain, Michael L.; Wasserman, Steven Alexander; Minorsky, Peter V.; Jackson, Robert Bradley (2008). Biology (8 ed.). San Francisco: Pearson – Benjamin Cummings. ISBN 978-0-321-54325-7.CS1 maint: ref=harv (link)
- McNeill, J.; et al., eds. (2012). International Code of Nomenclature for algae, fungi, and plants (Melbourne Code), Adopted by the Eighteenth International Botanical Congress Melbourne, Australia, July 2011 (electronic ed.). International Association for Plant Taxonomy. Retrieved 14 May 2017.
- Walsh PJ, Smith S, Fleming L, Solo-Gabriele H, Gerwick WH, eds. (2 September 2011). "Cyanobacteria and cyanobacterial toxins". Oceans and Human Health: Risks and Remedies from the Seas. Academic Press. pp. 271–296. ISBN 978-0-08-087782-2.
- "The Rise of Oxygen - Astrobiology Magazine". Astrobiology Magazine. Retrieved 6 April 2016.
- Flannery, D. T.; R.M. Walter (2012). "Archean tufted microbial mats and the Great Oxidation Event: new insights into an ancient problem". Australian Journal of Earth Sciences. 59 (1): 1–11. Bibcode:2012AuJES..59....1F. doi:10.1080/08120099.2011.607849.
- Rothschild, Lynn (September 2003). "Understand the evolutionary mechanisms and environmental limits of life". NASA. Archived from the original on 11 March 2012. Retrieved 13 July 2009.
- Nadis S (December 2003). "The cells that rule the seas" (PDF). Scientific American. 289 (6): 52–3. Bibcode:2003SciAm.289f..52N. doi:10.1038/scientificamerican1203-52. PMID 14631732. Archived from the original (PDF) on 19 April 2014. Retrieved 2 June 2019.
- "The Most Important Microbe You've Never Heard Of". npr.org.
- Flombaum, P.; Gallegos, J. L.; Gordillo, R. A.; Rincon, J.; Zabala, L. L.; Jiao, N.; Karl, D. M.; Li, W. K. W.; Lomas, M. W.; Veneziano, D.; Vera, C. S.; Vrugt, J. A.; Martiny, A. C. (2013). "Present and future global distributions of the marine Cyanobacteria Prochlorococcus and Synechococcus". Proceedings of the National Academy of Sciences. 110 (24): 9824–9829. Bibcode:2013PNAS..110.9824F. doi:10.1073/pnas.1307701110. PMC 3683724. PMID 23703908.
- Nabors, Murray W. (2004). Introduction to Botany. San Francisco, CA: Pearson Education, Inc. ISBN 978-0-8053-4416-5.
- Allaby, M., ed. (1992). "Algae". The Concise Dictionary of Botany. Oxford: Oxford University Press.
- Guiry MD (October 2012). "How many species of algae are there?". Journal of Phycology. 48 (5): 1057–63. doi:10.1111/j.1529-8817.2012.01222.x. PMID 27011267.
- Guiry, M.D.; Guiry, G.M. (2016). "Algaebase". www.algaebase.org. Retrieved 20 November 2016.
- D. Thomas (2002). Seaweeds. Life Series. Natural History Museum, London. ISBN 978-0-565-09175-0.
- Hoek, Christiaan; den Hoeck, Hoeck Van; Mann, David; Jahns, H.M. (1995). Algae : an introduction to phycology. Cambridge University Press. p. 166. ISBN 9780521316873. OCLC 443576944.
- Treguer, P.; Nelson, D. M.; Van Bennekom, A. J.; Demaster, D. J.; Leynaert, A.; Queguiner, B. (1995). "The Silica Balance in the World Ocean: A Reestimate". Science. 268 (5209): 375–9. Bibcode:1995Sci...268..375T. doi:10.1126/science.268.5209.375. PMID 17746543.
- "King's College London - Lake Megachad". www.kcl.ac.uk. Retrieved 5 May 2018.
- Gómez F (2012). "A checklist and classification of living dinoflagellates (Dinoflagellata, Alveolata)" (PDF). CICIMAR Océanides. 27 (1): 65–140. Archived from the original (PDF) on 27 November 2013.
- Stoecker DK (1999). "Mixotrophy among Dinoflagellates". The Journal of Eukaryotic Microbiology. 46 (4): 397–401. doi:10.1111/j.1550-7408.1999.tb04619.x.
- Starckx, Senne (31 October 2012) A place in the sun - Algae is the crop of the future, according to researchers in Geel Flanders Today, Retrieved 8 December 2012
- Duval, B.; Margulis, L. (1995). "The microbial community of Ophrydium versatile colonies: endosymbionts, residents, and tenants". Symbiosis. 18: 181–210. PMID 11539474.
- "Kelp Forest - an overview | ScienceDirect Topics". www.sciencedirect.com.
- Mann, K.H. (1973). "Seaweeds: their productivity and strategy for growth". Science. 182 (4116): 975–981. Bibcode:1973Sci...182..975M. doi:10.1126/science.182.4116.975. PMID 17833778.
- Tunnell, John Wesley; Chávez, Ernesto A.; Withers, Kim (2007). Coral reefs of the southern Gulf of Mexico. Texas A&M University Press. p. 91. ISBN 978-1-58544-617-9.
- Invasive Species Compendium: Caulerpa taxifolia (killer algae) Centre for Agriculture and Bioscience International. Updated: 6 November 2018.
- Mandoli, DF (1998). "Elaboration of Body Plan and Phase Change during Development of Acetabularia: How Is the Complex Architecture of a Giant Unicell Built?". Annual Review of Plant Physiology and Plant Molecular Biology. 49: 173–198. doi:10.1146/annurev.arplant.49.1.173. PMID 15012232.
- Pierre Madl; Maricela Yip (2004). "Literature Review of Caulerpa taxifolia". BUFUS-Info. 19 (31).
- Orth, R.J.; Carruthers, T.J.; Dennison, W.C.; Duarte, C.M.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; Kendrick, G.A.; Kenworthy, W.J.; Olyarnik, S.; Short, F.T. (2006). "A global crisis for seagrass ecosystems". BioScience. 56 (12): 987–996. doi:10.1641/0006-3568(2006)56[987:AGCFSE]2.0.CO;2. hdl:10261/88476.
- Froese, Rainer and Pauly, Daniel, eds. (2009). "Phycodurus eques" in FishBase. July 2009 version.
- Giri, C; Ochieng, E; Tieszen, LL; Zhu, Z; Singh, A; Loveland, T; et al. (2011). "Status and distribution of mangrove forests of the world using earth observation satellite data". Global Ecology and Biogeography. 20 (1): 154–159. doi:10.1111/j.1466-8238.2010.00584.x.
- Thomas, N.; Lucas, R.; Bunting, P.; Hardy, A.; Rosenqvist, A.; Simard, M. (2017). "Distribution and drivers of global mangrove forest change, 1996–2010". PLOS ONE. 12 (6): e0179302. Bibcode:2017PLoSO..1279302T. doi:10.1371/journal.pone.0179302.
- Short, F.T. and Frederick, T. (2003) World atlas of seagrasses, University of California Press, page 24. ISBN 9780520240476
- Lalli, C.; Parsons, T. (1993). Biological Oceanography: An Introduction. Butterworth-Heinemann. ISBN 0-7506-3384-0.
- Lindsey, R., Scott, M. and Simmon, R. (2010) "What are phytoplankton". NASA Earth Observatory.
- Field, C. B.; Behrenfeld, M. J.; Randerson, J. T.; Falwoski, P. G. (1998). "Primary production of the biosphere: Integrating terrestrial and oceanic components". Science. 281 (5374): 237–240. Bibcode:1998Sci...281..237F. doi:10.1126/science.281.5374.237. PMID 9657713.
- Rost, B. and Riebesell, U. (2004) "Coccolithophores and the biological pump: responses to environmental changes". In: Coccolithophores: From Molecular Processes to Global Impact, pages 99–125, Springer. ISBN 9783662062784.
- Arsenieff, L.; Simon, N.; Rigaut-Jalabert, F.; Le Gall, F.; Chaffron, S.; Corre, E.; Com, E.; Bigeard, E.; Baudoux, A.C. (2018). "First Viruses Infecting the Marine Diatom Guinardia delicatula". Frontiers in Microbiology. 9: 3235. doi:10.3389/fmicb.2018.03235. PMC 6334475. PMID 30687251.
- Varea, C.; Aragon, J.L.; Barrio, R.A. (1999). "Turing patterns on a sphere". Physical Review E. 60 (4): 4588. Bibcode:1999PhRvE..60.4588V. doi:10.1103/PhysRevE.60.4588.
- Harvey, Edmund Newton (1952). Bioluminescence. Academic Press.
- Suggested Explanation for Glowing Seas--Including Currently Glowing California Seas National Science Foundation, 18 October 2011.
- Castro P, Huber ME (2010). Marine Biology (8th ed.). McGraw Hill. p. 95. ISBN 978-0071113021.
- Hastings JW (1996). "Chemistries and colors of bioluminescent reactions: a review". Gene. 173 (1 Spec No): 5–11. doi:10.1016/0378-1119(95)00676-1. PMID 8707056.
- Haddock SH, Moline MA, Case JF (2009). "Bioluminescence in the sea". Annual Review of Marine Science. 2: 443–93. Bibcode:2010ARMS....2..443H. doi:10.1146/annurev-marine-120308-081028. PMID 21141672.
- U S Department of Energy (2008) Carbon Cycling and Biosequestration page 81, Workshop report DOE/SC-108, U.S. Department of Energy Office of Science.
- Campbell, Mike (22 June 2011). "The role of marine plankton in sequestration of carbon". EarthTimes. Retrieved 22 August 2014.
- Roman, J. & McCarthy, J.J. (2010). "The Whale Pump: Marine Mammals Enhance Primary Productivity in a Coastal Basin". PLoS ONE. 5 (10): e13255. Bibcode:2010PLoSO...513255R. doi:10.1371/journal.pone.0013255. PMC 2952594. PMID 20949007. e13255.CS1 maint: uses authors parameter (link)
- Brown, Joshua E. (12 October 2010). "Whale poop pumps up ocean health". Science Daily. Retrieved 18 August 2014.
- Thomson, Charles Wyville (2014) Voyage of the Challenger : The Atlantic Cambridge University Press, page235. ISBN 9781108074759.
- Grethe R. Hasle; Erik E. Syvertsen; Karen A. Steidinger; Karl Tangen (25 January 1996). "Marine Diatoms". In Carmelo R. Tomas (ed.). Identifying Marine Diatoms and Dinoflagellates. Academic Press. pp. 5–385. ISBN 978-0-08-053441-1. Retrieved 13 November 2013.
- Ald, S.M.; et al. (2007). "Diversity, Nomenclature, and Taxonomy of Protists" (PDF). Syst. Biol. 56 (4): 684–689. doi:10.1080/10635150701494127. PMID 17661235. Archived from the original (PDF) on 31 March 2011. Retrieved 1 October 2019.
- Moheimani, N.R.; Webb, J.P.; Borowitzka, M.A. (2012), "Bioremediation and other potential applications of coccolithophorid algae: A review. . Bioremediation and other potential applications of coccolithophorid algae: A review", Algal Research, 1 (2): 120–133, doi:10.1016/j.algal.2012.06.002
- Taylor, A.R.; Chrachri, A.; Wheeler, G.; Goddard, H.; Brownlee, C. (2011). "A voltage-gated H+ channel underlying pH homeostasis in calcifying coccolithophores". PLOS Biology. 9 (6): e1001085. doi:10.1371/journal.pbio.1001085. PMC 3119654. PMID 21713028.
- Prentice, I.C. (2001). "The carbon cycle and atmospheric carbon dioxide". Climate change 2001: the scientific basis: contribution of Working Group I to the Third Assessment Report of the Intergouvernmental Panel on Climate Change / Houghton, J.T. [edit.] Retrieved 31 May 2012.
- Halpern, B.S.; Frazier, M.; Afflerbach, J.; et al. (2019). "Recent pace of change in human impact on the world's ocean". Scientific Reports. 9 (1): 11609. Bibcode:2019NatSR...911609H. doi:10.1038/s41598-019-47201-9. PMC 6691109. PMID 31406130.
- Human impacts on marine ecosystems GEOMAR Helmholtz Centre for Ocean Research. Retrieved 22 October 2019.
- Rosing, M.; Bird, D.; Sleep, N.; Bjerrum, C. (2010). "No climate paradox under the faint early Sun". Nature. 464 (7289): 744–747. Bibcode:2010Natur.464..744R. doi:10.1038/nature08955. PMID 20360739.
- Sahney, S.; Benton, M.J.; Ferry, Paul (2010). "Links between global taxonomic diversity, ecological diversity and the expansion of vertebrates on land". Biology Letters. 6 (4): 544–7. doi:10.1098/rsbl.2009.1024. PMC 2936204. PMID 20106856.
- McKinney 1997, p. 110
- Stearns & Stearns 1999, p. x
- Novacek, Michael J. (8 November 2014). "Prehistory's Brilliant Future". The New York Times. New York: The New York Times Company. ISSN 0362-4331. Retrieved 25 December 2014.
- Nee, S. (2004). "Extinction, slime, and bottoms". PLoS Biology. 2 (8): E272. doi:10.1371/journal.pbio.0020272. PMC 509315. PMID 15314670.
- Ward, Peter D (2006). "Impact from the Deep". Scientific American. 295 (4): 64–71. Bibcode:2006SciAm.295d..64W. doi:10.1038/scientificamerican1006-64. PMID 16989482.
- Sahney, S. & Benton, M.J. (2008). "Recovery from the most profound mass extinction of all time" (PDF). Proceedings of the Royal Society B: Biological Sciences. 275 (1636): 759–65. doi:10.1098/rspb.2007.1370. PMC 2596898. PMID 18198148.
- Desmond, Adrian (1975). "The Discovery of Marine Transgressions and the Explanation of Fossils in Antiquity". American Journal of Science. 275 (6): 692–697. Bibcode:1975AmJS..275..692D. doi:10.2475/ajs.275.6.692.
- McKirahan, Richard D. "Xenophanes of Colophon. Philosophy Before Socrates. Indianapolis: Hackett Publishing Company, 1994. 66. Print.
- Grene MG, Grene M and Depew D (2004) The Philosophy of Biology: An Episodic History pages 1–34, Cambridge University Press. ISBN 9780521643801.
- Boylan, Michael "Aristotle: Biology" Internet Encyclopedia of Philosophy. Retrieved 5 August 2016.
- Lee, H. D. P. (1948) "Place-Names and the date of Aristotle's Biological Works". Classical Quarterly, 42 (3/4) :61–7
- Singer, Charles. A short history of biology. Oxford 1931.
- Emily Kearns, "Animals, knowledge about," in Oxford Classical Dictionary, 3rd ed., 1996, p. 92.
- Carl T. Bergstrom; Lee Alan Dugatkin (2012). Evolution. Norton. p. 35. ISBN 978-0-393-92592-0.
- Rhodes, Frank Harold Trevor (1 January 1974). Evolution. Golden Press. p. 7. ISBN 978-0-307-64360-5.
- Lalli, Carol M., and Timothy R. Parsons. "Introduction." Biological Oceanography: An Introduction. First Edition ed. Tarrytown, New York: Pergamon, 1993. 7-21. Print.
- Menden-Deuer, Susanne. "Course Info, OCG 561 Biological Oceanography".
- Miller, Charles B.; Patricia A. Wheeler (2012). Biological Oceanography (Second ed.). Chinchester, West Sussex: John Wiley & Sons.
Notes
- The earliest Bilateria may have had only a single opening, and no coelom.[242]
Further references
- Halpern, B.S.; Walbridge, S.; Selkoe, K.A.; Kappel, C.V.; Micheli, F.; D'Agrosa, C.; Bruno, J.F.; Casey, K.S.; Ebert, C.; Fox, H.E.; Fujita, R. (2008). "A global map of human impact on marine ecosystems". Science. 319 (5865): 948–952. Bibcode:2008Sci...319..948H. doi:10.1126/science.1149345. PMID 18276889.
- Paleczny, M.; Hammill, E.; Karpouzi, V.; Pauly, D. (2015). "Population trend of the world's monitored seabirds, 1950-2010". PLoS ONE. 10 (6): e0129342. Bibcode:2015PLoSO..1029342P. doi:10.1371/journal.pone.0129342. PMC 4461279. PMID 26058068.
- After 60 million years of extreme living, seabirds are crashing The Guardian, 22 September 2015.
- Ruppert, E.E.; Fox, R.S. & Barnes, R.D. (2004). Invertebrate Zoology (7th ed.). Brooks / Cole. ISBN 978-0-03-025982-1.
_-_Stephanopyxis_sp._-_630x_(14117161428).jpg)

.jpg)