Long QT syndrome
Long QT syndrome (LQTS) is a condition which affects repolarization of the heart after a heartbeat.[4] It results in an increased risk of an irregular heartbeat which can result in fainting, drowning, or sudden death.[1] These episodes can be triggered by exercise or stress.[5] Other associated symptoms may include hearing loss in certain types of long QT syndrome.[1]
| Long QT syndrome | |
|---|---|
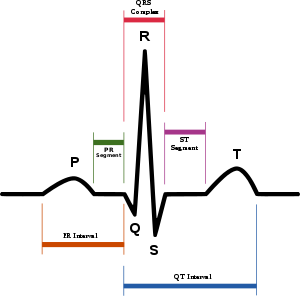 | |
| Drawing of a normal ECG tracing (sinus rhythm) with waves, segments, and intervals labeled. The QT interval is marked by the blue line at bottom. | |
| Specialty | Cardiology |
| Symptoms | Fainting, hearing loss, seizures[1] |
| Complications | Sudden death[1] |
| Causes | Genetic, certain medications, low blood potassium, low blood calcium, heart failure[2] |
| Risk factors | Family history of sudden death[3] |
| Diagnostic method | Electrocardiogram (EKG) together with clinical findings[4][3] |
| Differential diagnosis | Brugada syndrome, arrhythmogenic right ventricular dysplasia[3] |
| Treatment | Avoiding strenuous exercise, getting sufficient potassium, beta blockers, implantable cardiac defibrillator[5] |
| Frequency | ~ 1 in 7,000[5] |
| Deaths | ~3,500 a year (USA)[5] |
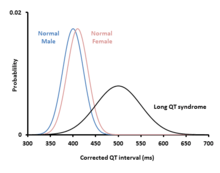
Long QT syndrome may be present at birth or develop later in life.[1] The inherited form may occur by itself or as part of larger genetic disorder.[1] Onset later in life may result from certain medications, low blood potassium, low blood calcium, or heart failure.[2] Medications that are implicated include certain antiarrhythmic, antibiotics, and antipsychotics.[2] Diagnosis is based on an electrocardiogram (EKG) finding a corrected QT interval of greater than 440 to 500 milliseconds together with clinical findings.[4][3]
Management may include avoiding strenuous exercise, getting sufficient potassium in the diet, the use of beta blockers, or an implantable cardiac defibrillator.[5] For people with LQTS who survive cardiac arrest and remain untreated, the risk of death within 15 years is greater than 50%.[6][5] With proper treatment this decreases to less than 1% over 20 years.[3]
Long QT syndrome is estimated to affect 1 in 7,000 people.[5] Females are affected more often than males.[5] Most people with the condition develop symptoms before they are 40 years old.[5] It is a relatively common cause of sudden death along with Brugada syndrome and arrhythmogenic right ventricular dysplasia.[3] In the United States it results in about 3,500 deaths a year.[5] The condition was first clearly described in 1957.[7]
Signs and symptoms
.jpg)
Many people with long QT syndrome have no signs or symptoms. Symptoms that do occur are generally caused by abnormal heart rhythms, most commonly a form of ventricular tachycardia called Torsades de pointes. If the arrhythmia reverts to a normal rhythm by itself then the affected person may experience lightheadedness (known as presyncope) or faint. These symptoms may be preceded by a fluttering sensation in the chest.[5] However, if the arrhythmia continues, the affected person may experience a cardiac arrest, which if untreated may lead to sudden death.[8] Those with LQTS may also experience seizure-like activity (non-epileptic seizure) as a result of reduced blood flow to the brain during an arrhythmia.[9][10] Epilepsy, however, is also associated with certain types of long QT syndrome.[10]
The arrhythmias that lead to faints and sudden death are more likely to occur in response to specific circumstances, in part determined by which genetic variant is responsible for the condition. While arrhythmias can occur at any time, in some forms of LQTS arrhythmias are more commonly seen in response to exercise or mental stress (LQT1), in other forms following a sudden loud noise (LQT2), and in some forms during sleep or immediately upon waking (LQT3).[8][11]
Some rare forms of long QT syndrome are associated with symptoms affecting other parts of the body. These include deafness in the Jervell and Lange-Nielsen form of the condition, and periodic paralysis in the Andersen–Tawil (LQT7) form.[12]
Risk for arrhythmias
While those with long QT syndrome have an increased risk of developing abnormal heart rhythms compared to those without the condition, the absolute risk of arrhythmias is very variable.[13] The strongest predictor of whether someone will develop torsades de pointes (TdP) is whether they have experienced spontaneous TdP or another form of cardiac arrest in the past.[14] Even if an arrhythmia has not been witnessed, a person with LQTS who has experienced syncope is also at higher risk, as syncope in these cases is frequently due to an undocumented self-terminating arrhythmia.[14]
In addition to a history of arrhythmias, the QT interval predicts risk. While some with LQTS will have QT intervals that are very prolonged, others will have only slight QT prolongation, or even a normal QT interval at rest (concealed LQTS). Those with the longest QT intervals are more likely to experience TdP, and a corrected QT interval of greater than 500 ms is thought to represent those at higher risk.[15] Despite this, even those with subtle QT prolongation or concealed LQTS still have some risk of arrhythmias.[8] Overall, every 10 ms increase in the corrected QT interval is associated with a 5% increase in arrhythmic risk.[13]
As the QT prolonging effects of both genetic variants and acquired causes of LQTS are additive, those with inherited LQTS are more likely to experience TdP if given QT prolonging drugs or develop electrolyte problems such as low potassium. Similarly, those taking QT prolonging medications are more likely to experience TdP if they have a genetic tendency to a prolonged QT interval, even it this tendency is concealed.[13] Arrhythmias occur more commonly in drug-induced LQTS if the medication in question has been rapidly given intravenously, or if high concentrations of the drug are present in the person's blood.[15] The risk of arrhythmias is also higher if the person receiving the drug has heart failure, is taking digitalis, or has recently been cardioverted from atrial fibrillation.[15] Other risk factors for developing torsades de pointes among those with LQTS include female sex, increasing age, pre-existing cardiovascular disease, and abnormal liver or kidney function.[16]
Causes
There are several subtypes of long QT syndrome. These can be broadly split into those caused by genetic mutations which those affected are born with, carry throughout their lives, and can pass on to their children (inherited or congenital long QT syndrome), and those caused by other factors which cannot be passed on and are often reversible (acquired long QT syndrome).
Inherited
Inherited, or congenital long QT syndrome, is caused by genetic abnormalities. LQTS can arise from variants in several genes, leading in some cases to quite different features.[17] However, the common thread linking these genetic variants is that they affect one or more ion currents leading to prolongation of the ventricular action potential (APD), thus lengthening the QT interval.[4] The condition is most commonly inherited in an autosomal dominant manner, or rarely in an autosomal recessive fashion.[18] Classification systems have been proposed to distinguish between subtypes of the condition based on the clinical features and subdivided by the underlying genetic variant that has caused the condition[19] The classical descriptions of forms of inherited LQTS based on clinical features are named after those who first described the condition. The commonest of these, accounting for 99% of cases, is Romano–Ward syndrome (genetically LQT1-6 and LQT9-16), an autosomal dominant form in which the electrical activity of the heart is affected without involvement of any other organs.[8] A less commonly seen form is Jervell and Lange-Nielsen syndrome, an autosomal recessive form of LQTS in which a combination of a prolonged QT interval and congenital deafness is seen.[20] Other rare forms include Anderson–Tawil syndrome (LQT7), which combines a prolonged QT interval with periodic paralysis and abnormalities of the face and skeleton; and Timothy syndrome (LQT8) which associates a prolonged QT interval with abnormalities in the structure of the heart and autism spectrum disorder.[12]
Romano–Ward syndrome
LQT1 is the most common subtype of Romano–Ward syndrome, responsible for 30 to 35% of all cases.[21] The gene responsible, KCNQ1, has been isolated to chromosome 11p15.5 and encodes the alpha subunit of the KvLQT1 potassium channel. This subunit interacts with other proteins (in particular, the minK beta subunit) to create the channel, which carries the delayed potassium rectifier current IKs responsible for the repolarisation phase of the cardiac action potential.[21] Variants in KCNQ1 cause the LQT1 subtype of Romano–Ward syndrome when a single copy of the variant is inherited (heterozygous, autosomal dominant inheritance). When two copies of the variant are inherited (homozygous, autosomal recessive inheritance) the more severe Jervell and Lange–Nielsen syndrome is found, associated with more marked QT prolongation, congenital sensorineural deafness, and a greater risk of arrhythmias.[21]
The LQT2 subtype is the second-most common form of Romano–Ward syndrome, responsible for 25 to 30% of all cases.[21] This form of Romano–Ward syndrome is caused by variants in the KCNH2 gene on chromosome 7.[21] KCNH2 (also known as hERG) encodes the potassium channel which carries the rapid inward rectifier current IKr. This current contributes to the terminal repolarisation phase of the cardiac action potential, and therefore the length of the QT interval.[21]
The LQT3 subtype of Romano–Ward syndrome is caused by variants in the SCN5A gene located on chromosome 3p21–24. SCN5A encodes the alpha subunit of the cardiac sodium channel, NaV1.5, responsible for the sodium current INa which depolarises cardiac cells at the start of the action potential.[21] Cardiac sodium channels normally inactivate rapidly, but the mutations involved in LQT3 slow their inactivation leading to a small sustained 'late' sodium current. This continued inward current prolongs the action potential and thereby the QT interval.[21] While some variants in SCN5A cause LQT3, other variants can cause quite different conditions. Variants causing a reduction in the early peak current can cause Brugada syndrome and cardiac conduction disease, while other variants have been associated with dilated cardiomyopathy. Some variants which affect both the early and late sodium current can cause overlap syndromes which combine aspects of both LQT3 and Brugada syndrome.[8]
Rare Romano–Ward subtypes (LQT4-6 and LQT9-16)
LQT5 is caused by variants in the KCNE1 gene. This gene is responsible for the potassium channel beta subunit MinK which, in conjunction with the alpha subunit encoded by KCNQ1, is responsible for the potassium current IKs, and variants associated with prolonged QT intervals decrease this current.[21] The same variants in KCNE1 can cause the more severe Jervell and Lange–Nielsen syndrome when two copies are inherited (homozygous inheritance) and the milder LQT5 subtype of Romano–Ward syndrome when a single copy of the variant is inherited (heterozygous inheritance).[22]
The LQT6 subtype is caused by variants in the KCNE2 gene.[21] This gene is responsible for the potassium channel beta subunit MiRP1 which generates the potassium current IKr, and variant that decrease this current have been associated with prolongation of the QT interval.[22] However, subsequent evidence such as the relatively common finding of variants in the gene in those without long QT syndrome, and the general need for a second stressor such as hypokalaemia to be present to reveal the QT prolongation, has suggested that this gene instead represents a modifier to susceptibility to QT prolongation.[19] Some therefore dispute whether variants in the gene are sufficient to cause Romano-Ward syndrome by themselves.[19]
LQT9 is caused by variants in the membrane structural protein, caveolin-3.[21] Caveolins form specific membrane domains called caveolae in which voltage-gated sodium channels sit. Similar to LQT3, these caveolin variants increase the late sustained sodium current, which impairs cellular repolarization.[21]
LQT10 is an extremely rare subtype, caused by variants in the SCN4B gene. The product of this gene is an auxiliary beta-subunit (NaVβ4) forming cardiac sodium channels, variants in which increase the late sustained sodium current.[21] LQT13 is caused by variants in GIRK4, a protein involved in the parasympathetic modulation of the heart.[21] Clinically, the patients are characterized by only modest QT prolongation, but an increased propensity for atrial arrhythmias. LQT14, LQT15 and LQT16 are caused by variants in the genes responsible for calmodulin (CALM1, CALM2, and CALM3 respectively).[21] Calmodulin interacts with several ion channels and its roles include modulation of the L-type calcium current in response to calcium concentrations, and trafficking the proteins produced by KCNQ1 and thereby influencing potassium currents.[21] The precise mechanisms by which means these genetic variants prolong the QT interval remain uncertain.[21]
Andersen–Tawil syndrome (LQT7)
LQT7, also known as Andersen–Tawil syndrome, is characterised by a triad of features – in addition to a prolonged QT interval, those affected may experience intermittent weakness often occurring at times when blood potassium concentrations are low (hypokalaemic periodic paralysis), and characteristic facial and skeletal abnormalities such as a small lower jaw (micrognathia), low set ears, and fused or abnormally angled fingers and toes (syndactyly and clinodactyly). The condition is inherited in an autosomal-dominant manner and is frequently associated with mutations in the KCNJ2 gene which encodes the potassium channel protein Kir2.1.[23]
Timothy syndrome (LQT8)
Timothy syndrome is due to mutations in the calcium channel Cav1.2 encoded by the gene CACNA1c. Since the calcium channel Cav1.2 is abundant in many tissues, patients with Timothy syndrome have many clinical manifestations, including other congenital heart disease, autism, immune deficiency and complex syndactyly.[24]
Table of associated genes
The following is a list of genes associated with LQTS:
| Type | OMIM | Gene | Notes |
| LQT1 | 192500 | KCNQ1 | Encodes the α-subunit of the slow delayed rectifier potassium channel KV7.1 carrying the potassium current IKs.[19] |
| LQT2 | 152427 | KCNH2 | Also known as hERG. Encodes the α-subunit of the rapid delayed rectifier potassium channel KV11.1 carrying the potassium current IKr.[19] |
| LQT3 | 603830 | SCN5A | Encodes the α-subunit of the cardiac sodium channel NaV1.5 carrying the sodium current INa.[19] |
| LQT4 | 600919 | ANK2 | Encodes Ankyrin B which anchors the ion channels in the cell. Disputed whether truly disease causing versus minor QT susceptibility gene.[19] |
| LQT5 | 176261 | KCNE1 | Encodes MinK, a potassium channel β-subunit. Heterozygous inheritance causes Romano–Ward, homozygous inheritance causes Jervell and Lange–Nielsen syndrome.[19] |
| LQT6 | 603796 | KCNE2 | Encodes MiRP1, a potassium channel β-subunit. Disputed whether truly disease causing versus minor QT susceptibility gene.[19] |
| LQT7 | 170390 | KCNJ2 | Encodes inward rectifying potassium current Kir2.1 carrying the potassium current IK1. Causes Andersen–Tawil syndrome.[19] |
| LQT8 | 601005 | CACNA1c | Encodes the α-subunit CaV1.2 of the calcium channel Cav1.2 carrying the calcium current ICa(L). Causes Timothy syndrome.[19] |
| LQT9 | 611818 | CAV3 | Encodes Caveolin-3, responsible for forming membrane pouches known as caveolae. Mutations in this gene may increase the late sodium current INa.[19] |
| LQT10 | 611819 | SCN4B | Encodes the β4-subunit of the cardiac sodium channel.[19] |
| LQT11 | 611820 | AKAP9 | Encodes A-kinase associated protein which interacts with KV7.1.[19] |
| LQT12 | 601017 | SNTA1 | Encodes syntrophin-α1. Mutations in this gene may increase the late sodium current INa.[19] |
| LQT13 | 600734 | KCNJ5 | Also known as GIRK4, encodes G protein-sensitive inwardly rectifying potassium channels (Kir3.4) which carry the potassium current IK(ACh).[19] |
| LQT14 | 616247 | CALM1 | Encodes calmodulin-1, a calcium-binding messenger protein that interacts with the calcium current ICa(L).[19] |
| LQT15 | 616249 | CALM2 | Encodes calmodulin-2, a calcium-binding messenger protein that interacts with the calcium current ICa(L).[19] |
| LQT16 | 114183 | CALM3 | Encodes calmodulin-3, a calcium-binding messenger protein that interacts with the calcium current ICa(L).[19] |
Acquired
Although long QT syndrome is often a genetic condition, a prolonged QT interval associated with an increased risk of abnormal heart rhythms can also occur in people without a genetic abnormality, commonly due to a side effect of medications. Drug-induced QT prolongation is often a result of treatment by antiarrhythmic drugs such as amiodarone and sotalol, antibiotics such as erythromycin, or antihistamines such as terfenadine.[16] Other drugs which prolong the QT interval include some antipsychotics such as haloperidol and ziprasidone, and the antidepressant citalopram.[25][15] Lists of medications associated with prolongation of the QT interval such as the CredibleMeds database can be found online.[26]
Other causes of acquired LQTS include abnormally low levels of potassium (hypokalaemia) or magnesium (hypomagnesaemia) within the blood. This is can be exacerbated following a sudden reduction in the blood supply to the heart (myocardial infarction), low levels of thyroid hormone (hypothyroidism), and a slow heart rate (bradycardia).[27]
Anorexia nervosa has been associated with sudden death, possibly due to QT prolongation. The malnutrition seen in this condition can sometimes affect the blood concentration of salts such as potassium, potentially leading to acquired long QT syndrome, in turn causing sudden cardiac death. The malnutrition and associated changes in salt balance develop over a prolonged period of time, and rapid refeeding may further disturb the salt imbalances, increasing the risk of arrhythmias. Care must therefore be taken to monitor electrolyte levels to avoid the complications of refeeding syndrome.[28]
Factors which prolong the QT interval are additive, and genetic variants may make those who carry them more susceptible to drug-induced LQT. A combination of factors can cause a greater degree of QT prolongation than each factor alone.[27]
Mechanisms
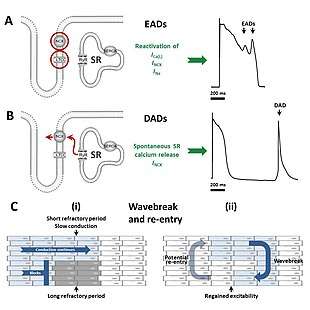
The various forms of long QT syndrome, both congenital and acquired, lead to a tendency to abnormal heart rhythms through their effects on the electrical signals used to coordinate individual heart cells. The common theme linking the various causes is a prolongation of the cardiac action potential – the characteristic pattern of voltage changes across the cell membrane that occur with each heart beat.[8] Heart cells when relaxed normally have fewer positively charged ions on the inner side of their cell membrane than on the outer side, referred to as the membrane being polarised. When heart cells contract, positively charged ions such as sodium and calcium enter the cell, equalising or reversing this polarity, at which point the cell is said to be depolarised. After a contraction has taken place, the cell restores its polarity (or repolarises) by allowing positively charged ions such as potassium to leave the cell, restoring the membrane to its relaxed, polarised state. In long QT syndrome it takes longer for this repolarisation to occur, shown in individual cells as a longer action potential while being marked on the surface ECG as a long QT interval.[8]
The prolonged action potentials can lead to arrhythmias through several potential mechanisms. The characteristic arrhythmia seen in long QT syndrome, Torsades de Pointes, starts when additional action potentials occur after an initial triggering beat in the form of afterdepolarisations. Early afterdepolarisations, occurring before the cell has fully repolarised, are particularly likely to be seen when action potentials are prolonged, and arise due to reactivation of calcium and sodium channels that would normally switch off until the next heartbeat is due.[29] Under the right conditions, reactivation of these currents, facilitated by the sodium-calcium exchanger, can cause further depolarisation of the cell.[29] The early afterdepolarisations triggering arrhythmias in long QT syndrome tend to arise from the Purkinje fibres of the cardiac conduction system.[30] Early afterdepolarisations may occur as single events, but may occur repeatedly leading to multiple rapid activations of the cell.[29]
Some research suggests that delayed afterdepolarisations, occurring after repolarisation has completed, may also play a role in long QT syndrome.[30] This form of afterdepolarisation originates from the spontaneous release of calcium from the intracellular calcium store known as the sarcoplasmic reticulum, forcing calcium out of cell through the sodium calcium exchanger in exchange for sodium, generating a net inward current.[29]
While there is strong evidence that the trigger for Torsades de Pointes comes from afterdepolarisations, it is less certain what sustains this arrhythmia. Some lines of evidence suggest that repeated afterdepolarisations from many sources contribute to the continuing arrhythmia.[30] However, some suggest that the arrhythmia sustains through a mechanism known as re-entry. According to this model, the action potential prolongation occurs to a variable extent in different layers of the heart muscle with longer action potentials in some layers than others.[30] In response to a triggering impulse, the waves of depolarisation will spread through regions with shorter action potentials but block in regions with longer action potentials. This allows the depolarising wavefront to bend around areas of block, potentially forming a complete loop and self-perpetuating. The twisting pattern on the ECG can be explained by movement of the core of the re-entrant circuit in the form of a meandering spiral wave.[30]
Diagnosis
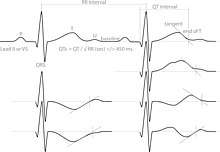
Diagnosing long QT syndrome is challenging. The QT interval is highly variable among both those who are healthy and those who have LQTS. This leads to overlap between the QT intervals of those with LQTS and those without – 2.5% of those with LQTS have a normal QT interval while 10–15% of the healthy population has a prolonged QT.[31] Other factors beyond the QT interval should be taken into account when making a diagnosis, some of which have been incorporated into scoring systems.[12]
Schwartz score
The Schwartz score has been proposed as a method of combining clinical and ECG factors to assess how likely an individual is to have an inherited form of LQTS.[4] The table below lists the criteria used to calculate the score.
| Schwartz score to aid diagnosis of inherited long QT syndrome. [32] | |||
| Corrected QT interval (QTc) | ≥ 480 ms | 3 points | QTc defined according to Bazett's correction |
| 460–470 ms | 2 points | ||
| 450 ms and male gender | 1 point | ||
| Torsades de pointes | 2 points | ||
| T-wave alternans | 1 point | ||
| Notched T-waves in at least 3 leads | 1 point | ||
| Low heart rate for age (children) | 0.5 points | ||
| Syncope | with stress | 2 points | Cannot receive points both for syncope and Torsades |
| without stress | 1 point | ||
| Congenital deafness | 0.5 points | ||
| Family history | Other family member with confirmed LQTS | 1 point | Same family member cannot be counted for LQTS and sudden death |
| Sudden cardiac death in immediate family member aged <30 | 0.5 point | ||
| Score: 0–1 : low probability of LQTS; 2–3 : intermediate probability of LQTS; ≥ 4 : high probability of LQTS | |||
Other investigations
In cases of diagnostic uncertainty, other investigations designed to unmask a prolonged QT may be helpful. In addition to its effect on the QT interval at rest, LQTS affects how the QT changes in response to exercise and stimulation by catecholamines such as adrenaline. Provocation tests, in the form of exercise tolerance tests or direct infusion of adrenaline, can be used to detect these abnormal responses.[33] These investigations are most useful for identifying the group of those with congenital LQTS type 1 (LQT1) who have a normal QT interval at rest. In these people, exercise or adrenaline infusion can lead to paradoxical prolongation of the QT interval, revealing the underlying condition.[20]
Guideline cutoffs
The European Society of Cardiology recommend that LQTS can be diagnosed if the corrected QT duration is longer than 480ms, or if an individual has a Schwartz score of greater than 3. They recommend that a diagnosis can also be made if a pathogenic genetic variant associated with LQTS is identified, regardless of QT interval. Furthermore, a diagnosis can be considered in the presence of a QTC of greater than 460ms if unexplained syncope has occurred.[12]
Treatment
Those diagnosed with LQTS are usually advised to avoid drugs that can prolong the QT interval further or lower the threshold for TDP, lists of which can be found in public access online databases.[34] In addition to this, two intervention options are known for individuals with LQTS: arrhythmia prevention and arrhythmia termination.
Arrhythmia prevention
Arrhythmia suppression involves the use of medications or surgical procedures that attack the underlying cause of the arrhythmias associated with LQTS. Since the cause of arrhythmias in LQTS is early afterdepolarizations (EADs), and they are increased in states of adrenergic stimulation, steps can be taken to blunt adrenergic stimulation in these individuals. These include administration of beta receptor blocking agents, which decreases the risk of stress-induced arrhythmias. Beta blockers are an effective treatment for LQTS caused by LQT1 and LQT2.[4]
Genotype and QT interval duration are independent predictors of recurrence of life-threatening events during beta-blocker therapy. To be specific, the presence of QTc >500 ms and LQT2 and LQT3 genotype are associated with the highest incidence of recurrence. In these patients, primary prevention with use of implantable cardioverter-defibrillators can be considered.[4]
- Potassium supplementation: If the potassium content in the blood rises, the action potential shortens, so increasing potassium concentration could minimize the occurrence of arrhythmias. It should work best in LQT2, since the hERG channel is especially sensitive to potassium concentration, but the use is experimental and not evidence-based.
- Sodium channel blocking drugs such as mexiletine have been used to prevent arrhythmias in long QT syndrome.[35] While the most compelling indication is for those whose long QT syndrome is caused by defective sodium channels producing a sustained late current (LQT3), mexiletine also shortens the QT interval in other forms of long QT syndrome including LQT1, LQT2 and LQT8.[35] As the predominant action of mexiletine is on the early peak sodium current, there are theoretical reasons why drugs which preferentially suppress the late sodium current such as ranolazine may be more effective, although evidence that this is the case in practice is limited.[35]
- Amputation of the cervical sympathetic chain (left stellectomy). This therapy is typically reserved for LQTS caused by JLNS,[4] but may be used as an add-on therapy to beta blockers in certain cases. In most cases, modern therapy favors ICD implantation if beta blocker therapy fails.
Arrhythmia termination
Arrhythmia termination involves stopping a life-threatening arrhythmia once it has already occurred. One effective form of arrhythmia termination in individuals with LQTS is placement of an implantable cardioverter-defibrillator (ICD). Also, external defibrillation can be used to restore sinus rhythm. ICDs are commonly used in patients with fainting episodes despite beta blocker therapy, and in patients having experienced a cardiac arrest.
With better knowledge of the genetics underlying LQTS, more precise treatments hopefully will become available.[36]
Outcomes
For people who experience cardiac arrest or fainting caused by LQTS and who are untreated, the risk of death within 15 years is around 50%.[6] With careful treatment this decreases to less than 1% over 20 years.[3] Those who exhibit symptoms before the age of 18 are more likely to experience a cardiac arrest.[20][37]
Epidemiology
Inherited LQTS is estimated to affect between one in 2,500 and 7,000 people.[4]
History
The first documented case of LQTS was described in Leipzig by Meissner in 1856, when a deaf girl died after her teacher yelled at her. Soon after being notified, the girl's parents reported that her older brother, also deaf, had previously died after a terrible fright.[38] This was several decades before the ECG was invented, but is likely the first described case of Jervell and Lange-Nielsen syndrome. In 1957, the first case documented by ECG was described by Anton Jervell and Fred Lange-Nielsen, working in Tønsberg, Norway.[39] Italian pediatrician Cesarino Romano, in 1963,[40] and Irish pediatrician Owen Conor Ward, in 1964,[41] separately described the more common variant of LQTS with normal hearing, later called Romano-Ward syndrome. The establishment of the International Long-QT Syndrome Registry in 1979 allowed numerous pedigrees to be evaluated in a comprehensive manner. This helped in detecting many of the numerous genes involved.[42]
References
- "Long QT syndrome". Genetic and Rare Diseases Information Center (GARD) – an NCATS Program. 2017. Retrieved 14 December 2017.
- Morita H, Wu J, Zipes DP (August 2008). "The QT syndromes: long and short". Lancet. 372 (9640): 750–63. doi:10.1016/S0140-6736(08)61307-0. PMID 18761222.
- Ferri FF (2016). Ferri's Clinical Advisor 2017 E-Book: 5 Books in 1. Elsevier Health Sciences. p. 736. ISBN 9780323448383.
- Levine E, Rosero SZ, Budzikowski AS, Moss AJ, Zareba W, Daubert JP (August 2008). "Congenital long QT syndrome: considerations for primary care physicians". Cleveland Clinic Journal of Medicine. 75 (8): 591–600. doi:10.3949/ccjm.75.8.591. PMID 18756841.
- "Long QT Syndrome". NHLBI, NIH. Retrieved 14 December 2017.
- Ackerman MJ, Priori SG, Dubin AM, Kowey P, Linker NJ, Slotwiner D, et al. (January 2017). "Beta-blocker therapy for long QT syndrome and catecholaminergic polymorphic ventricular tachycardia: Are all beta-blockers equivalent?". Heart Rhythm. 14 (1): e41–e44. doi:10.1016/j.hrthm.2016.09.012. PMID 27659101.
Among patients who have experienced a LQTS-triggered cardiac event (arrhythmic syncope, arrhythmic syncope followed by seizures, or aborted cardiac arrest), the untreated natural history is grim, with >50% mortality at 15 years.

- Vincent J, Abraham E, Kochanek P, Moore FA, Fink MP (2011). Textbook of Critical Care E-Book. Elsevier Health Sciences. p. 578. ISBN 978-1437715682.
- Tester DJ, Schwartz PJ, Ackerman MJ (2013). "Congenital Long QT Syndrome". In Gussak I, Antzelevitch C (eds.). Electrical Diseases of the Heart. London: Springer. pp. = 39–46. doi:10.1007/978-1-4471-4881-4_27. ISBN 978-1-4471-4881-4.
- McMillan, Julia A.; Feigin, Ralph D.; DeAngelis, Catherine; Jones, M. Douglas (2006). Oski's Pediatrics: Principles & Practice. Lippincott Williams & Wilkins. p. 1677. ISBN 978-0-7817-3894-1.
- Madan, Nandini; Carvalho, Karen S. (February 2017). "Neurological Complications of Cardiac Disease". Seminars in Pediatric Neurology. 24 (1): 3–13. doi:10.1016/j.spen.2017.01.001. ISSN 1558-0776. PMID 28779863.
Syncope can lead to convulsions and can easily be confused with epileptic seizures.
- Nakajima T, Kaneko Y, Kurabayashi M (2015). "Unveiling specific triggers and precipitating factors for fatal cardiac events in inherited arrhythmia syndromes". Circulation Journal. 79 (6): 1185–92. doi:10.1253/circj.CJ-15-0322. PMID 25925977.
- Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. (November 2015). "2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC)Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC)". Europace. 17 (11): 1601–87. doi:10.1093/europace/euv319. PMID 26318695.
- Trinkley KE, Page RL, Lien H, Yamanouye K, Tisdale JE (December 2013). "QT interval prolongation and the risk of torsades de pointes: essentials for clinicians". Current Medical Research and Opinion. 29 (12): 1719–26. doi:10.1185/03007995.2013.840568. PMID 24020938.
- Barsheshet A, Dotsenko O, Goldenberg I (November 2013). "Genotype-specific risk stratification and management of patients with long QT syndrome". Annals of Noninvasive Electrocardiology. 18 (6): 499–509. doi:10.1111/anec.12117. PMC 6932574. PMID 24206565.
- Roden DM (March 2004). "Drug-induced prolongation of the QT interval". The New England Journal of Medicine. 350 (10): 1013–22. doi:10.1056/NEJMra032426. PMID 14999113.
- Thomson C, Wright P (2014-10-15). "Long QT syndrome". The Pharmaceutical Journal. 293 (7833). Retrieved 18 October 2014.
- Hedley PL, Jørgensen P, Schlamowitz S, Wangari R, Moolman-Smook J, Brink PA, et al. (November 2009). "The genetic basis of long QT and short QT syndromes: a mutation update". Human Mutation. 30 (11): 1486–511. doi:10.1002/humu.21106. PMID 19862833.
- Goldman 2011, pp. 185
- Giudicessi JR, Wilde AA, Ackerman MJ (October 2018). "The genetic architecture of long QT syndrome: A critical reappraisal". Trends in Cardiovascular Medicine. 28 (7): 453–464. doi:10.1016/j.tcm.2018.03.003. PMC 6590899. PMID 29661707.
- Giudicessi JR, Ackerman MJ (October 2013). "Genotype- and phenotype-guided management of congenital long QT syndrome". Current Problems in Cardiology. 38 (10): 417–55. doi:10.1016/j.cpcardiol.2013.08.001. PMC 3940076. PMID 24093767.
- Bohnen MS, Peng G, Robey SH, Terrenoire C, Iyer V, Sampson KJ, Kass RS (January 2017). "Molecular Pathophysiology of Congenital Long QT Syndrome". Physiological Reviews. 97 (1): 89–134. doi:10.1152/physrev.00008.2016. PMC 5539372. PMID 27807201.
- Giudicessi JR, Ackerman MJ (October 2013). "Genotype- and phenotype-guided management of congenital long QT syndrome". Current Problems in Cardiology. 38 (10): 417–55. doi:10.1016/j.cpcardiol.2013.08.001. PMC 3940076. PMID 24093767.
- Nguyen HL, Pieper GH, Wilders R (December 2013). "Andersen–Tawil syndrome: clinical and molecular aspects". International Journal of Cardiology. 170 (1): 1–16. doi:10.1016/j.ijcard.2013.10.010. PMID 24383070.
- Tristani-Firouzi, Martin; Etheridge, Susan P. (2013), Gussak, Ihor; Antzelevitch, Charles (eds.), "Andersen–Tawil and Timothy Syndromes", Electrical Diseases of the Heart, Springer London, pp. 561–567, doi:10.1007/978-1-4471-4881-4_32, ISBN 978-1-4471-4880-7
- Beach SR, Celano CM, Noseworthy PA, Januzzi JL, Huffman JC (January 2013). "QTc prolongation, torsades de pointes, and psychotropic medications". Psychosomatics. 54 (1): 1–13. doi:10.1016/j.psym.2012.11.001. PMID 23295003.
- Woosley RL, Black K, Heise CW, Romero K (February 2018). "CredibleMeds.org: What does it offer?" (PDF). Trends in Cardiovascular Medicine. 28 (2): 94–99. doi:10.1016/j.tcm.2017.07.010. hdl:10150/627826. PMID 28801207.
- El-Sherif N, Turitto G, Boutjdir M (April 2018). "Acquired long QT syndrome and torsade de pointes". Pacing and Clinical Electrophysiology. 41 (4): 414–421. doi:10.1111/pace.13296. PMID 29405316.
- Jáuregui-Garrido B, Jáuregui-Lobera I (February 2012). "Sudden death in eating disorders". Vascular Health and Risk Management. 8: 91–8. doi:10.2147/VHRM.S28652. PMC 3292410. PMID 22393299.
- Wit AL (June 2018). "Afterdepolarizations and triggered activity as a mechanism for clinical arrhythmias". Pacing and Clinical Electrophysiology. 41 (8): 883–896. doi:10.1111/pace.13419. PMID 29920724.
- El-Sherif N, Turitto G, Boutjdir M (March 2019). "Acquired Long QT Syndrome and Electrophysiology of Torsade de Pointes". Arrhythmia & Electrophysiology Review. 8 (2): 122–130. doi:10.15420/aer.2019.8.3. PMC 6528034. PMID 31114687.
- Moric-Janiszewska E, Markiewicz-Łoskot G, Łoskot M, Weglarz L, Hollek A, Szydłowski L (September 2007). "Challenges of diagnosis of long-QT syndrome in children". Pacing and Clinical Electrophysiology. 30 (9): 1168–70. doi:10.1111/j.1540-8159.2007.00832.x. PMID 17725765.
- Schwartz PJ, Moss AJ, Vincent GM, Crampton RS (August 1993). "Diagnostic criteria for the long QT syndrome. An update". Circulation. 88 (2): 782–4. doi:10.1161/01.CIR.88.2.782. PMID 8339437.
- Obeyesekere MN, Klein GJ, Modi S, Leong-Sit P, Gula LJ, Yee R, et al. (December 2011). "How to perform and interpret provocative testing for the diagnosis of Brugada syndrome, long-QT syndrome, and catecholaminergic polymorphic ventricular tachycardia". Circulation: Arrhythmia and Electrophysiology. 4 (6): 958–64. doi:10.1161/CIRCEP.111.965947. PMID 22203660.
- "QT Drug List by Risk Groups". Arizona Center for Education and Research on Therapeutics. Archived from the original on 2010-12-24. Retrieved 2010-07-04.
- Li G, Zhang L (November 2018). "The role of mexiletine in the management of long QT syndrome". Journal of Electrocardiology. 51 (6): 1061–1065. doi:10.1016/j.jelectrocard.2018.08.035. PMID 30497731.
- Compton SJ, Lux RL, Ramsey MR, Strelich KR, Sanguinetti MC, Green LS, et al. (September 1996). "Genetically defined therapy of inherited long-QT syndrome. Correction of abnormal repolarization by potassium". Circulation. 94 (5): 1018–22. doi:10.1161/01.CIR.94.5.1018. PMID 8790040.
- "Genotype risk relationship".
- Tranebjaerg L, Bathen J, Tyson J, Bitner-Glindzicz M (September 1999). "Jervell and Lange-Nielsen syndrome: a Norwegian perspective". American Journal of Medical Genetics. 89 (3): 137–46. doi:10.1002/(SICI)1096-8628(19990924)89:3<137::AID-AJMG4>3.0.CO;2-C. PMID 10704188.
- Jervell A, Lange-Nielsen F (July 1957). "Congenital deaf-mutism, functional heart disease with prolongation of the Q-T interval and sudden death". American Heart Journal. 54 (1): 59–68. doi:10.1016/0002-8703(57)90079-0. PMID 13435203.
- Romano C, Gemme G, Pongiglione R (September 1963). "[Arrythmias of the Pediatric Age. II. Syncopal Attacks Due to Paroxysmal Ventricular Fibrillation. (Presentation of 1st Case in Italian Pediatric Literature]". La Clinica Pediatrica (in Italian). 45: 656–83. PMID 14158288.
- Ward OC (April 1964). "A New Familial Cardiac Syndrome in Children". Journal of the Irish Medical Association. 54: 103–6. PMID 14136838.
- Moss AJ, Schwartz PJ (March 2005). "25th anniversary of the International Long-QT Syndrome Registry: an ongoing quest to uncover the secrets of long-QT syndrome". Circulation. 111 (9): 1199–201. doi:10.1161/01.CIR.0000157069.91834.DA. PMID 15753228.
- Notes
- Goldman L (2011). Goldman's Cecil Medicine (24th ed.). Philadelphia: Elsevier Saunders. p. 1196. ISBN 978-1437727883.CS1 maint: ref=harv (link)
External links
| Classification | |
|---|---|
| External resources |
|
- CredibleMeds.org, contains a list of drugs that prolong the QT interval