Linoleic acid
Linoleic acid (LA) is a polyunsaturated omega-6 fatty acid and is one of two essential fatty acids for humans, who must obtain it through their diet.[4] It is a colorless or white oil that is virtually insoluble in water.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(9Z,12Z)-octadeca-9,12-dienoic acid | |
| Other names
cis,cis-9,12-octadecadienoic acid C18:2 (Lipid numbers) | |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | |
Beilstein Reference |
1727101 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.428 |
| EC Number |
|
Gmelin Reference |
57557 |
IUPHAR/BPS |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
| C18H32O2 | |
| Molar mass | 280.452 g·mol−1 |
| Appearance | Colorless oil |
| Density | 0.9 g/cm3[1] |
| Melting point | −12 °C (10 °F)[1] −6.9 °C (19.6 °F)[2] −5 °C (23 °F)[3] |
| Boiling point | 229 °C (444 °F) at 16 mmHg[2] 230 °C (446 °F) at 21 mbar[3] 230 °C (446 °F) at 16 mmHg[1] |
| 0.139 mg/L[3] | |
| Vapor pressure | 16 Torr at 229 °C |
| Hazards | |
| NFPA 704 (fire diamond) | 
1
2
0 |
| Flash point | 112 °C (234 °F)[3] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The word "linoleic" derives from the Greek word linon (flax). Oleic means "of, relating to, or derived from oil of olive" or "of or relating to oleic acid" because saturating the omega-6 double bond produces oleic acid.
Chemistry
Linoleic acid is a fatty acid. It is an 18-carbon chain with two double bonds in cis configuration. A shorthand notation like "18:2 (n-6)" or "18:2 cis-9,12" may be used in literature.[5] It typically occurs in nature as a triglyceride ester; free fatty acids, the form not combined with glycerol to form triglyceride, are typically low in foods.[6] It is very soluble in acetone, benzene, diethyl ether and ethanol.[2]
In physiology
Linoleic acid is a polyunsaturated fatty acid used in the biosynthesis of arachidonic acid (AA) with elongation and saturation, [7] and thus some prostaglandins, leukotrienes (LTA, LTB, LTC), and thromboxane (TXA). It is found in the lipids of cell membranes. It is abundant in many nuts, fatty seeds (flax seeds, hemp seeds, poppy seeds, sesame seeds, etc.) and their derived vegetable oils; comprising over half (by weight) of poppy seed, safflower, sunflower, corn, and soybean oils.[8]
The consumption of linoleic acid is vital to proper health, as it is an essential fatty acid.[9] In rats, a diet deficient in linoleate (the salt form of the acid) has been shown to cause mild skin scaling, hair loss,[10] and poor wound healing.[11] However, chronic consumption of high levels of LA may be associated with the development of ulcerative colitis.[12]
Cockroaches release oleic and linoleic acid upon death, which prevents other roaches from entering the area. This is similar to the mechanism found in ants and bees, which release oleic acid upon death.[13]
Metabolism and eicosanoids
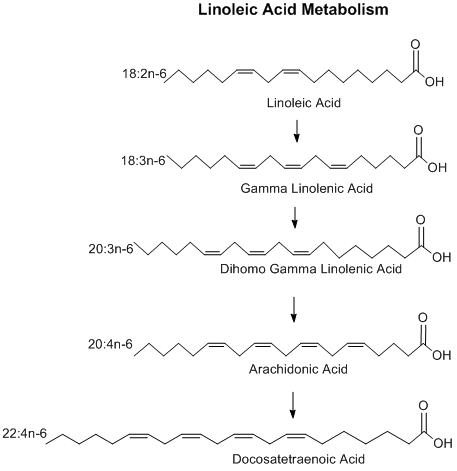
The first step in the metabolism of linoleic acid is performed by Δ6desaturase, which converts LA into gamma-Linolenic acid (GLA).
There is evidence suggesting that infants lack Δ6desaturase of their own, and must acquire it through breast milk. Studies show that breast-milk fed babies have higher concentrations of GLA than formula-fed babies, while formula-fed babies have elevated concentrations of LA.[14]
GLA is converted to dihomo-γ-linolenic acid (DGLA), which in turn is converted to arachidonic acid (AA). One of the possible fates of AA is to be transformed into a group of metabolites called eicosanoids during the inflammatory response and during physical activity; eicosanoids are a class of paracrine hormones. The three types of eicosanoids are prostaglandins, thromboxanes, and leukotrienes. Eicosanoids produced from AA tend to promote (not cause) inflammation and promote growth during and after physical activity in healthy humans.[15] For example, both AA-derived thrombaxane and leukotrieneB4 are proaggregatory and vasoconstrictive eicosanoids during inflammation. The oxidized metabolic products of linoleic acid, such as 9-hydroxyoctadecanoic acid and 13-hydroxyoctadecanoic acid, have also been shown to activate TRPV1, the capsaicin receptor, and through this might play a major role in hyperalgesia and allodynia.[16]
There are some suggested negative health effects related to this inflammation promoting function of linoleic acid as an omega-6 fatty acid.
In addition, LA is converted by various lipoxygenases, cyclooxygenases, certain cytochrome P450 enzymes (the CYP monooxygenases), and non-enzymatic autoxidation mechanisms to mono-hydroxyl products viz., 13-Hydroxyoctadecadienoic acid and 9-Hydroxyoctadecadienoic acid; these two hydroxy metabolites are enzymatically oxidized to their keto metabolites, 13-oxo-octadecadienoic acid and 9-oxo-octadecdienoic acid. Certain cytochrome P450 enzymes, the CYP epoxygenases, metabolize LA to epoxide products viz., its 12,13-epoxide, Vernolic acid and its 9,10-epoxide, Coronaric acid. All of these linoleic acid products have bioactivity and are implicated in human physiology and pathology as indicated in the cited linkages.
Uses
Industrial uses
Linoleic acid is used in making quick-drying oils, which are useful in oil paints and varnishes. These applications exploit the easy reaction of the linoleic acid with oxygen in air, which leads to crosslinking and formation of a stable film called linoxyn.
Reduction of linoleic acid yields linoleyl alcohol. Linoleic acid is a surfactant with a critical micelle concentration of 1.5 x 10−4 M @ pH 7.5.
Linoleic acid has become increasingly popular in the beauty products industry because of its beneficial properties on the skin. Research points to linoleic acid's anti-inflammatory, acne reductive, skin-lightening and moisture retentive properties when applied topically on the skin.[17][18][19][20]
Use in research
Linoleic acid lipid radicals can be used to show the antioxidant effect of polyphenols and natural phenols. Experiments on linoleic acid subjected to 2,2'-Azobis(2-amidinopropane) dihydrochloride induced oxidation of linoleic acid; hence producing lipid radicals and then the use of different combinations of phenolics show that binary mixtures can lead to either a synergetic antioxidant effect or to an antagonistic effect towards the lipid radicals. Research like this is useful in discovering which phenols prevent the autoxidation of lipids in vegetable oils.[21]
Dietary sources
| Name | % LA† | ref. |
|---|---|---|
| Salicornia oil | 75% | |
| Safflower oil | 74.62% | |
| Evening Primrose oil | 65-80% | [22] |
| Melon seed oil | 70% | |
| Poppyseed oil | 70% | |
| Grape seed oil | 69.6% | |
| Sunflower oil | 65.7% | |
| Prickly Pear seed oil | 62.3% | |
| Barbary Fig seed oil | 65% | |
| Hemp oil | 54.3% | [23] |
| Corn oil | 59% | |
| Wheat germ oil | 55% | |
| Cottonseed oil | 54% | |
| Soybean oil | 51% | |
| Walnut oil | 51% | |
| Sesame oil | 45% | |
| Rice bran oil | 39% | |
| Argan oil | 37% | |
| Pistachio oil | 32.7% | |
| Peanut oil | 32% | [24] |
| Peach oil | 29% | [25] |
| Almonds | 24% | |
| Canola oil | 21% | |
| Chicken fat | 18-23% | [26] |
| Egg yolk | 16% | |
| Linseed oil (flax) | 15% | |
| Lard | 10% | |
| Olive oil | 10% (3.5 - 21%) | [27][28] |
| Palm oil | 10% | |
| Durio graveolens | 4.95% | [29] |
| Cocoa butter | 3% | |
| Macadamia oil | 2% | |
| Butter | 2% | |
| Coconut oil | 2% | |
| †average val | ||
See also
- Conjugated linoleic acid
- Omega-6 fatty acid: Negative health effects
- Essential fatty acids
- Essential fatty acid interactions
- Eicosanoids
- Essential nutrients
- Linolein
References
- The Merck Index, 11th Edition, 5382
- William M. Haynes (2016). CRC Handbook of Chemistry and Physics (97th ed.). Boca Raton: CRC Press. p. 3–338. ISBN 978-1-4987-5429-3.
- Record of CAS RN 60-33-3 in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- Simopoulos, Artemis P. (2008). "The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases". Experimental Biology and Medicine. 233 (6): 674–688. doi:10.3181/0711-mr-311. PMID 18408140.
- "Fatty Acids". Cyber Lipid. Archived from the original on 28 October 2018. Retrieved 31 July 2017.
- Mattes, RD (2009). "Is there a fatty acid taste?". Annu. Rev. Nutr. 29: 305–27. doi:10.1146/annurev-nutr-080508-141108. PMC 2843518. PMID 19400700.
- Whelan J, Fritsche K. (2013). "Linoleic acid". Adv Nutr. 4 (3): 311–312. doi:10.3945/an.113.003772. PMC 3650500. PMID 23674797.CS1 maint: uses authors parameter (link)
- "Nutrient Data Laboratory Home Page". USDA National Nutrient Database for Standard Reference, Release 20. U.S. Department of Agriculture, Agricultural Research Service. 2007. Archived from the original on 14 April 2016.
- Whelan, Jay; Fritsche, Kevin (May 2013). "Linoleic Acid". Advances in Nutrition. 4 (3): 311–312. doi:10.3945/an.113.003772. PMC 3650500. PMID 23674797.
- Cunnane S, Anderson M (1 April 1997). "Pure linoleate deficiency in the rat: influence on growth, accumulation of n-6 polyunsaturates, and (1-14C) linoleate oxidation". J Lipid Res. 38 (4): 805–12. PMID 9144095. Retrieved 15 January 2007.
- Ruthig DJ, Meckling-Gill KA (1 October 1999). "Both (n-3) and (n-6) fatty acids stimulate wound healing in the rat intestinal epithelial cell line, IEC-6". Journal of Nutrition. 129 (10): 1791–8. doi:10.1093/jn/129.10.1791. PMID 10498749.
- "Role of Fats in Ulcerative Colitis | Gastrointestinal Society". www.badgut.org. Retrieved 9 January 2018.
- "Earth News: Ancient 'smell of death' revealed". BBC. 9 September 2009.
- David F. Horrobin (1993). "Fatty acid metabolism in health and disease: the role of Δ-6-desaturase". American Journal of Clinical Nutrition. 57 (5 Suppl): 732S–7S. doi:10.1093/ajcn/57.5.732S. PMID 8386433.
- Piomelli, Daniele (2000). "Arachidonic Acid". Neuropsychopharmacology: The Fifth Generation of Progress. Archived from the original on 15 July 2006. Retrieved 16 April 2009.
- Patwardhan, AM; Scotland, PE; Akopian, AN; Hargreaves, KM (2009). "Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia". Proceedings of the National Academy of Sciences of the United States of America. 106 (44): 18820–4. Bibcode:2009PNAS..10618820P. doi:10.1073/pnas.0905415106. PMC 2764734. PMID 19843694.
- Diezel, W.E.; Schulz, E.; Skanks, M.; Heise, H. (1993). "Plant oils: Topical application and anti-inflammatory effects (croton oil test)". Dermatologische Monatsschrift. 179: 173.
- Letawe, C; Boone, M; Pierard, GE (March 1998). "Digital image analysis of the effect of topically applied linoleic acid on acne microcomedones". Clinical and Experimental Dermatology. 23 (2): 56–58. doi:10.1046/j.1365-2230.1998.00315.x. PMID 9692305.
- Ando, H; Ryu, A; Hashimoto, A; Oka, M; Ichihashi, M (March 1998). "Linoleic acid and α-linolenic acid lightens ultraviolet-induced hyperpigmentation of the skin". Archives of Dermatological Research. 290 (7): 375–381. doi:10.1007/s004030050320. PMID 9749992.
- Darmstadt, GL; Mao-Qiang, M; Chi, E; Saha, SK; Ziboh, VA; Black, RE; Santosham, M; Elias, PM (2002). "Impact of topical oils on the skin barrier: possible implications for neonatal health in developing countries". Acta Paediatrica. 91 (5): 546–554. CiteSeerX 10.1.1.475.1064. doi:10.1080/080352502753711678. PMID 12113324.
- Peyrat-Maillard, M. N.; Cuvelier, M. E.; Berset, C. (2003). "Antioxidant activity of phenolic compounds in 2,2′-azobis (2-amidinopropane) dihydrochloride (AAPH)-induced oxidation: Synergistic and antagonistic effects". Journal of the American Oil Chemists' Society. 80 (10): 1007–1012. doi:10.1007/s11746-003-0812-z.
- "Evening Primrose Oil for Menopause does it help". 26 January 2018.
- Oomah, B. Dave; Busson, Muriel; Godfrey, David V; Drover, John C. G (1 January 2002). "Characteristics of hemp (Cannabis sativa L.) seed oil". Food Chemistry. 76 (1): 33–43. doi:10.1016/S0308-8146(01)00245-X.
- Oil, peanut, salad or cooking: search for peanut oil on "USDA Food Composition Databases". Archived from the original on 3 March 2015. Retrieved 16 September 2009.
- Wu, Hao; Shi, John; Xue, Sophia; Kakuda, Yukio; Wang, Dongfeng; Jiang, Yueming; Ye, Xingqian; Li, Yanjun; Subramanian, Jayasankar (2011). "Essential oil extracted from peach (Prunus persica) kernel and its physicochemical and antioxidant properties". LWT - Food Science and Technology. 44 (10): 2032–2039. doi:10.1016/j.lwt.2011.05.012.
- M. K. Nutter, E. E. Lockhart and R. S. Harris (1943). "The chemical composition of depot fats in chickens and turkeys". Journal of the American Oil Chemists' Society. 20 (11): 231–234. doi:10.1007/BF02630880.
- "Olive Oil : Chemical Characteristics".
- Beltran; Del Rio, C; Sánchez, S; Martínez, L (2004). "Influence of Harvest Date and Crop Yield on the Fatty Acid Composition of Virgin Olive Oils from Cv. Picual" (PDF). J. Agric. Food Chem. 52 (11): 3434–3440. doi:10.1021/jf049894n. PMID 15161211.
- Nasaruddin, Mohd hanif; Noor, Noor Qhairul Izzreen Mohd; Mamat, Hasmadi (2013). "Komposisi Proksimat dan Komponen Asid Lemak Durian Kuning (Durio graveolens) Sabah" [Proximate and Fatty Acid Composition of Sabah Yellow Durian (Durio graveolens)] (PDF). Sains Malaysiana (in Malay). 42 (9): 1283–1288. ISSN 0126-6039. OCLC 857479186. Retrieved 28 November 2017.
Further reading
- "Compound Summary: Linoleic acid". PubChem. U.S. National Library of Medicine.
External links
- Linoleic acid MS Spectrum
- Fatty Acids: Methylene-Interrupted Double Bonds, AOCS Lipid Library
