Foraminifera
Foraminifera (/fəˌræməˈnɪfərə/; Latin for "hole bearers"; informally called "forams") are members of a phylum or class of amoeboid protists characterized by streaming granular ectoplasm for catching food and other uses; and commonly an external shell (called a "test") of diverse forms and materials. Tests of chitin (found in some simple genera, and Textularia in particular) are believed to be the most primitive type. Most foraminifera are marine, the majority of which live on or within the seafloor sediment (i.e., are benthic), while a smaller variety float in the water column at various depths (i.e., are planktonic). Fewer are known from freshwater or brackish conditions, and some very few (nonaquatic) soil species have been identified through molecular analysis of small subunit ribosomal DNA.[2][3]
| Foraminifera | |
|---|---|
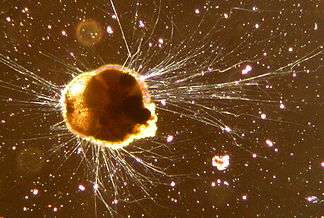 | |
| Live Ammonia tepida (Rotaliida) | |
| Scientific classification | |
| Domain: | |
| (unranked): | |
| (unranked): | Rhizaria |
| Phylum: | Retaria |
| Subphylum: | Foraminifera d'Orbigny, 1826 |
| Orders | |
|
Allogromiida | |
Foraminifera typically produce a test, or shell, which can have either one or multiple chambers, some becoming quite elaborate in structure.[4] These shells are commonly made of calcium carbonate (CaCO
3) or agglutinated sediment particles. Over 50,000 species are recognized, both living (10,000)[5] and fossil (40,000).[6][7] They are usually less than 1 mm in size, but some are much larger, the largest species reaching up to 20 cm.[8]
In modern scientific English, the term foraminifera is both singular and plural (irrespective of the word's Latin derivation), and is used to describe one or more specimens or taxa: its usage as singular or plural must be determined from context. Foraminifera is frequently used informally to describe the group, and in these cases is generally lowercase.[9]
Taxonomy
Alcide d'Orbigny, in his 1826 work, considered them to be a group of minute cephalopods and noted their odd morphology, interpreting the pseudopodia as tentacles and noting the highly reduced (in actuality, absent) head. [10] He named the group foraminifères, or "hole-bearers", as members of the group had holes in the divisions between compartments in their shells, in contrast to nautili or ammonites. [9]
The taxonomic position of the Foraminifera has varied since their recognition as protozoa (protists) by Schultze in 1854,[11] there referred to as an order, Foraminiferida. Loeblich and Tappan (1992) reranked Foraminifera as a class[12] as it is now commonly regarded.
The Foraminifera have typically been included in the Protozoa,[13][14][15] or in the similar Protoctista or Protist kingdom.[16][17] Compelling evidence, based primarily on molecular phylogenetics, exists for their belonging to a major group within the Protozoa known as the Rhizaria.[13] Prior to the recognition of evolutionary relationships among the members of the Rhizaria, the Foraminifera were generally grouped with other amoeboids as phylum Rhizopodea (or Sarcodina) in the class Granuloreticulosa.
The Rhizaria are problematic, as they are often called a "supergroup", rather than using an established taxonomic rank such as phylum. Cavalier-Smith defines the Rhizaria as an infra-kingdom within the kingdom Protozoa.[13]
Some taxonomies put the Foraminifera in a phylum of their own, putting them on par with the amoeboid Sarcodina in which they had been placed.
Although as yet unsupported by morphological correlates, molecular data strongly suggest the Foraminifera are closely related to the Cercozoa and Radiolaria, both of which also include amoeboids with complex shells; these three groups make up the Rhizaria.[14] However, the exact relationships of the forams to the other groups and to one another are still not entirely clear. Foraminifera are closely related to testate amoebae.[18]
| Taxonomy from Mikhalevich 2013[19] |
|---|
* Foraminifera d'Orbigny 1826
|
Living Foraminifera
Modern Foraminifera are primarily marine organisms, but living individuals have been found in brackish, freshwater [20] and even terrestrial habitats.[3] The majority of the species are benthic, and a further 40 morphospecies are planktonic.[21] This count may, however, represent only a fraction of actual diversity, since many genetically distinct species may be morphologically indistinguishable.[22]
A number of forams have unicellular algae as endosymbionts, from diverse lineages such as the green algae, red algae, golden algae, diatoms, and dinoflagellates.[21] These mixotrophic foraminifers are particularly common in nutrient-poor oceanic waters.[23] Some forams are kleptoplastic, retaining chloroplasts from ingested algae to conduct photosynthesis.[24]
Biology

The foraminiferal cell is divided into granular endoplasm and transparent ectoplasm from which a pseudopodial net may emerge through a single opening or through many perforations in the test. Individual pseudopods characteristically have small granules streaming in both directions.[20] The pseudopods are used for locomotion, anchoring, excretion, test construction and in capturing food, which consists of small organisms such as diatoms or bacteria.[21][25] Certain foraminifera prey upon small animals such as copepods or cumaceans; some forams even predate upon other forams, drilling holes into the tests of their prey.[26]
The generalized foraminiferal life-cycle involves an alternation between haploid and diploid generations, although they are mostly similar in form.[11][27] The haploid or gamont initially has a single nucleus, and divides to produce numerous gametes, which typically have two flagella. The diploid or schizont is multinucleate, and after meiosis divides to produce new gamonts. Multiple rounds of asexual reproduction between sexual generations are not uncommon in benthic forms.[20]
Certain benthic foraminifera have been found to be capable of surviving anoxic conditions for over 24 hours, indicating that they are capable of selective anaerobic respiration. This is interpreted as an adaptation to survive changing oxygenic conditions near the sediment-water interface. [28]
Tests
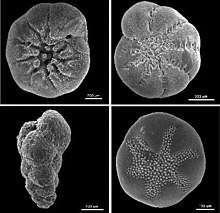


The form and composition of their tests are the primary means by which forams are identified and classified. Most secrete calcareous tests, composed of calcium carbonate.[20] Calcareous tests may be composed of either aragonite or calcite depending on species; among those with calcite tests, the test may contain either a high or low fraction of magnesium substitution.[29] In other forams, the tests may be composed of organic material, made from small pieces of sediment cemented together (agglutinated) by either proteins or calcium carbonate, and in one genus, of silica. Some studies suggest a high amount of homoplasy in foraminifera, and that neither agglutinated nor calcareous foraminifera form monophyletic groupings.[30]
The test contains an organic matrix, which can sometimes be recovered from fossil samples.[29]
Openings in the test that allow the cytoplasm to extend outside are called apertures. [31] The primary aperture, leading to the exterior, take many different shapes in different species, including but not limited to rounded, crescent-shaped, slit-shaped, hooded, radiate (star-shaped), dendritic (branching). Some foraminifera have "toothed", flanged, or lipped primary apertures. There may be only one primary aperture or multiple; when multiple are present, they may be clustered or equatorial. In addition to the primary aperture, many foraminifera have supplemental apertures. These may form as relict apertures (past primary apertures from an earlier growth stage) or as unique structures.
Test shape is highly variable among different foraminifera; they may be single-chambered (unilocular) or multi-chambered (multilocular). In multilocular forms, new chambers are added as the organism grows. A wide variety of test morphologies is found in both unilocular and multilocular forms, including spiraled, serial, and milioline, among others. [25]
Unlike other shell-secreting organisms, such as molluscs or corals, the tests of foraminifera are located inside the cell membrane, within the protoplasm. The organelles of the cell are located within the compartment(s) of the test, and the hole(s) of the test allow the transfer of material from the pseudopodia to the internal cell and back. [25]
Tests as fossils are known from as far back as the Ediacaran period, [32]and many marine sediments are composed primarily of them. For instance, the limestone that makes up the pyramids of Egypt is composed almost entirely of nummulitic benthic Foraminifera.[33] It is estimated that reef Foraminifera generate about 43 million tons of calcium carbonate per year.[34]

Genetic studies have identified the naked amoeba Reticulomyxa and the peculiar xenophyophores as foraminiferans without tests. A few other amoeboids produce reticulose pseudopods, and were formerly classified with the forams as the Granuloreticulosa, but this is no longer considered a natural group, and most are now placed among the Cercozoa.[35]
Deep-sea species
Foraminifera are found in the deepest parts of the ocean such as the Mariana Trench, including the Challenger Deep, the deepest part known. At these depths, below the carbonate compensation depth, the calcium carbonate of the tests is soluble in water due to the extreme pressure. The Foraminifera found in the Challenger Deep thus have no carbonate test, but instead have one of organic material.[36]
Four species found in the Challenger Deep are unknown from any other place in the oceans, one of which is representative of an endemic genus unique to the region. They are Resigella laevis and R. bilocularis, Nodellum aculeata, and Conicotheca nigrans (the unique genus). All have tests that are mainly of transparent organic material which have small (about 100 nm) plates that appear to be clay.[36]
Evolutionary significance

Dying planktonic Foraminifera continuously rain down on the sea floor in vast numbers, their mineralized tests preserved as fossils in the accumulating sediment. Beginning in the 1960s, and largely under the auspices of the Deep Sea Drilling, Ocean Drilling, and International Ocean Drilling Programmes, as well as for the purposes of oil exploration, advanced deep-sea drilling techniques have been bringing up sediment cores bearing Foraminifera fossils.[37] The effectively unlimited supply of these fossil tests and the relatively high-precision age-control models available for cores has produced an exceptionally high-quality planktonic Foraminifera fossil record dating back to the mid-Jurassic, and presents an unparalleled record for scientists testing and documenting the evolutionary process.[37] The exceptional quality of the fossil record has allowed an impressively detailed picture of species inter-relationships to be developed on the basis of fossils, in many cases subsequently validated independently through molecular genetic studies on extant specimens[38] Larger benthic Foraminifera with complex shell structure react in a highly specific manner to the different benthic environments and, therefore, the composition of the assemblages and the distribution patterns of particular species reflect simultaneously bottom types and the light gradient. In the course of Earth history, larger Foraminifera are replaced frequently. In particular, associations of Foraminifera characterizing particular shallow water facies types are dying out and are replaced after a certain time interval by new associations with the same structure of shell morphology, emerging from a new evolutionary process of adaptation.[39] These evolutionary processes make the larger Foraminifera useful as index fossils for the Permian, Jurassic, Cretaceous and Cenozoic.
The earliest known definite foraminifera appear in the fossil record towards the very end of the Ediacaran; these forms all have agglutinated tests and are unilocular. These include forms like Platysolenites and Spirosolenites. [40][32] Molecular clocks indicate that the crown-group of foraminifera likely evolved during the Neoproterozoic or latest Mesoproterozoic; this timing is consistent with Neoproterozoic fossils of the closely-related filose amoebae. As fossils of foraminifera have not been found prior to the end of the Ediacaran, it is likely that most of these Proterozoic forms did not have hard-shelled tests.[41]
Uses
Because of their diversity, abundance, and complex morphology, fossil foraminiferal assemblages are useful for biostratigraphy, and can accurately give relative dates to sedimentary rocks, as was discovered by Alva C. Ellisor in 1920.[42] The oil industry relies heavily on microfossils such as forams to find potential hydrocarbon deposits.[43]
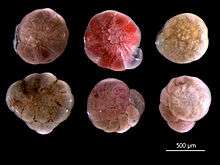
Calcareous fossil Foraminifera are formed from elements found in the ancient seas where they lived. Thus, they are very useful in paleoclimatology and paleoceanography. They can be used, as a climate proxy, to reconstruct past climate by examining the stable isotope ratios and trace element content of the shells (tests). Global temperature and ice volume can be revealed by the isotopes of oxygen, and the history of the carbon cycle and oceanic productivity by examining the stable isotope ratios of carbon;[44] see δ18O and δ13C. The concentration of trace elements, like magnesium (Mg),[45] lithium (Li)[46] and boron (B),[47] also hold a wealth of information about global temperature cycles, continental weathering, and the role of the ocean in the global carbon cycle. Geographic patterns seen in the fossil records of planktonic forams are also used to reconstruct ancient ocean currents. Because certain types of Foraminifera are found only in certain environments, they can be used to figure out the kind of environment under which ancient marine sediments were deposited. For the same reasons they make useful biostratigraphic markers, living foraminiferal assemblages have been used as bioindicators in coastal environments, including indicators of coral reef health. Because calcium carbonate is susceptible to dissolution in acidic conditions, Foraminifera may be particularly affected by changing climate and ocean acidification.

Foraminifera have many uses in petroleum exploration and are used routinely to interpret the ages and paleoenvironments of sedimentary strata in oil wells.[48] Agglutinated fossil Foraminifera buried deeply in sedimentary basins can be used to estimate thermal maturity, which is a key factor for petroleum generation. The Foraminiferal Colouration Index [49] (FCI) is used to quantify colour changes and estimate burial temperature. FCI data is particularly useful in the early stages of petroleum generation (about 100 °C).
Foraminifera can also be used in archaeology in the provenancing of some stone raw material types. Some stone types, such as limestone, are commonly found to contain fossilised Foraminifera. The types and concentrations of these fossils within a sample of stone can be used to match that sample to a source known to contain the same "fossil signature".
Gallery
 Foraminifera of Pag Island, Adriatic Sea -60 m, field width 5.5 mm
Foraminifera of Pag Island, Adriatic Sea -60 m, field width 5.5 mm Foraminifera of Pag Island, Adriatic Sea -60 m, field width 5.5 mm
Foraminifera of Pag Island, Adriatic Sea -60 m, field width 5.5 mm Foraminifera of Pag Island, Adriatic Sea -60 m, field width 5.5 mm
Foraminifera of Pag Island, Adriatic Sea -60 m, field width 5.5 mm Foraminifera of Pag Island, Adriatic Sea -60 m, field width 5.5 mm
Foraminifera of Pag Island, Adriatic Sea -60 m, field width 5.5 mm Foraminifera of Indian Ocean, south-eastern coast of Bali, field width 5.5 mm
Foraminifera of Indian Ocean, south-eastern coast of Bali, field width 5.5 mm Foraminifera of Indian Ocean, south-eastern coast of Bali, field width 5.5 mm
Foraminifera of Indian Ocean, south-eastern coast of Bali, field width 5.5 mm Foraminifera of Indian Ocean, south-eastern coast of Bali, field width 5.5 mm
Foraminifera of Indian Ocean, south-eastern coast of Bali, field width 5.5 mm Foraminifera in Ngapali, Myanmar, field width 5.22 mm
Foraminifera in Ngapali, Myanmar, field width 5.22 mm Foraminifera Heterostegina depressa, field width 4.4 mm
Foraminifera Heterostegina depressa, field width 4.4 mm
References
- Parfrey, Laura Wegener; Lahr, Daniel J. G.; Knoll, Andrew H.; Katz, Laura A. (16 August 2011). "Estimating the timing of early eukaryotic diversification with multigene molecular clocks". Proceedings of the National Academy of Sciences of the United States of America. 108 (33): 13624–13629. Bibcode:2011PNAS..10813624P. doi:10.1073/pnas.1110633108. PMC 3158185. PMID 21810989.
- Giere, Olav (2009). Meiobenthology: the microscopic motile fauna of aquatic sediments (2nd ed.). Berlin: Springer. ISBN 978-3540686576.
- Lejzerowicz, Franck; Pawlowski, Jan; Fraissinet-Tachet, Laurence; Marmeisse, Roland (1 September 2010). "Molecular evidence for widespread occurrence of Foraminifera in soils". Environmental Microbiology. 12 (9): 2518–26. doi:10.1111/j.1462-2920.2010.02225.x. PMID 20406290.
- Kennett, J.P.; Srinivasan, M.S. (1983). Neogene planktonic foraminifera: a phylogenetic atlas. Hutchinson Ross. ISBN 978-0-87933-070-5.
- Ald, S.M. et al. (2007) Diversity, Nomenclature, and Taxonomy of Protists, Syst. Biol. 56(4), 684–689, DOI: 10.1080/10635150701494127.
- Pawlowski, J., Lejzerowicz, F., & Esling, P. (2014). Next-generation environmental diversity surveys of foraminifera: preparing the future. The Biological Bulletin, 227(2), 93-106.
- "World Foraminifera Database".
- Marshall M (3 February 2010). "Zoologger: 'Living beach ball' is giant single cell". New Scientist.
- Lipps JH, Finger KL, Walker SE (October 2011). "What Should We call the Foraminifera" (PDF). Journal of Foraminiferal Research. 41 (4): 309–313. doi:10.2113/gsjfr.41.4.309. Retrieved 10 April 2018.
- d'Orbigny, Alcide (1826). "Tableau Méthodique de la Classe des Céphalopodes". Annales des Sciences Naturelles, Paris (Série 1). 7: 245–314. – via Biodiversity Heritage Library.
- Loeblich Jr, A.R.; Tappan, H. (1964). "Foraminiferida". Part C, Protista 2. Treatise on Invertebrate Paleontology. Geological Society of America. pp. C55–C786. ISBN 978-0-8137-3003-5.
- Sen Gupta, Barun K. (2002). Modern Foraminifera. Springer. p. 16. ISBN 978-1-4020-0598-5.
- Cavalier-Smith, T (2004). "Only Six Kingdoms of Life" (PDF).
- Cavalier-Smith, T (2003). "Protist phylogeny and the high-level classification of Protozoa". European Journal of Protistology. 34 (4): 338–348. doi:10.1078/0932-4739-00002.
- Tolweb Cercozoa
- European Register of Marine Species
- eForams-taxonomy Archived 3 October 2011 at the Wayback Machine
- Testate amoebae as environmental indicators (PDF), archived from the original (PDF) on 27 November 2016, retrieved 27 November 2016
- Mikhalevich, V.I. (2013). "New insight into the systematics and evolution of the foraminifera". Micropaleontology. 59 (6): 493–527.
- Sen Gupta, Barun K. (1982). "Ecology of benthic Foraminifera". In Broadhead, T.W. (ed.). Foraminifera: notes for a short course organized by M.A. Buzas and B.K. Sen Gupta. Studies in Geology. 6. University of Tennessee, Dept. of Geological Sciences. pp. 37–50. ISBN 978-0910249058. OCLC 9276403.
- Hemleben, C.; Anderson, O.R.; Spindler, M. (1989). Modern Planktonic Foraminifera. Springer-Verlag. ISBN 978-3-540-96815-3.
- Kucera, M.; Darling, K.F. (April 2002). "Cryptic species of planktonic foraminifera: their effect on palaeoceanographic reconstructions". Philos Trans Royal Soc A. 360 (1793): 695–718. Bibcode:2002RSPTA.360..695K. doi:10.1098/rsta.2001.0962. PMID 12804300.
- Advances in Microbial Ecology, Volum 11
- Bernhard, J. M.; Bowser, S.M. (1999). "Benthic Foraminifera of dysoxic sediments: chloroplast sequestration and functional morphology". Earth-Science Reviews. 46 (1): 149–165. Bibcode:1999ESRv...46..149B. doi:10.1016/S0012-8252(99)00017-3.
- Saraswati, Pratul Kumar; Srinivasan, M. S. (2016), Saraswati, Pratul Kumar; Srinivasan, M.S. (eds.), "Calcareous-Walled Microfossils", Micropaleontology: Principles and Applications, Springer International Publishing, pp. 81–119, doi:10.1007/978-3-319-14574-7_6, ISBN 978-3-319-14574-7, retrieved 3 April 2020
- Goldstein, Susan T. (2003), Sen Gupta, Barun K. (ed.), "Foraminifera: A biological overview", Modern Foraminifera, Springer Netherlands, pp. 37–55, doi:10.1007/0-306-48104-9_3, ISBN 978-0-306-48104-8, retrieved 3 April 2020
- Moore, R.C.; Lalicker, A.G.; Fischer, C.G. (1952). "Ch 2 Foraminifera and Radiolaria". Invertebrate Fossils. McGraw-Hill. OCLC 547380.
- Moodley, L.; Hess, C. (1 August 1992). "Tolerance of Infaunal Benthic Foraminifera for Low and High Oxygen Concentrations". The Biological Bulletin. 183 (1): 94–98. doi:10.2307/1542410. ISSN 0006-3185.
- Sen Gupta, Barun K. (2003), Sen Gupta, Barun K. (ed.), "Systematics of moder Foraminifera", Modern Foraminifera, Springer Netherlands, pp. 7–36, doi:10.1007/0-306-48104-9_2, ISBN 978-0-306-48104-8, retrieved 3 April 2020
- Pawlowski, Jan; Holzmann, Maria; Tyszka, Jarosław (1 April 2013). "New supraordinal classification of Foraminifera: Molecules meet morphology". Marine Micropaleontology. 100: 1–10. doi:10.1016/j.marmicro.2013.04.002. ISSN 0377-8398.
- Lana, C (2001). "Cretaceous Carterina (Foraminifera)". Marine Micropaleontology. 41 (1–2): 97–102. Bibcode:2001MarMP..41...97L. doi:10.1016/S0377-8398(00)00050-5.
- Kontorovich, A. E.; Varlamov, A. I.; Grazhdankin, D. V.; Karlova, G. A.; Klets, A. G.; Kontorovich, V. A.; Saraev, S. V.; Terleev, A. A.; Belyaev, S. Yu.; Varaksina, I. V.; Efimov, A. S. (1 December 2008). "A section of Vendian in the east of West Siberian Plate (based on data from the Borehole Vostok 3)". Russian Geology and Geophysics. 49 (12): 932–939. doi:10.1016/j.rgg.2008.06.012. ISSN 1068-7971.
- Foraminifera: History of Study, University College London, retrieved 20 September 2007
- Langer, M. R.; Silk, M. T. B.; Lipps, J. H. (1997). "Global ocean carbonate and carbon dioxide production: The role of reef Foraminifera". Journal of Foraminiferal Research. 27 (4): 271–277. doi:10.2113/gsjfr.27.4.271.
- Adl, S. M.; Simpson, A. G. B.; Farmer, M. A.; Anderson; et al. (2005). "The new higher level classification of Eukaryotes with emphasis on the taxonomy of Protists". Journal of Eukaryotic Microbiology. 52 (5): 399–451. doi:10.1111/j.1550-7408.2005.00053.x. PMID 16248873.
- Gooday, A.J.; Todo, Y.; Uematsu, K.; Kitazato, H. (July 2008). "New organic-walled Foraminifera (Protista) from the ocean's deepest point, the Challenger Deep (western Pacific Ocean)". Zoological Journal of the Linnean Society. 153 (3): 399–423. doi:10.1111/j.1096-3642.2008.00393.x.
- "Nature debates".
- Journal bioinformatics and biology insights, Using the Multiple Analysis Approach to Reconstruct Phylogenetic Relationships among Planktonic Foraminifera from Highly Divergent and Length-polymorphic SSU rDNA Sequences
- Omaña, L.; Alencaster, G.; Buitrón, B.E. (2016). "Mid-early late Albian foraminiferal assemblage from the El Abra Formation in the El Madroño locality, eastern Valles–San Luis Potosí Platform, Mexico: Paleoenvironmental and paleobiogeographical significance". Boletín de la Sociedad Geológica Mexicana. 68 (3): 477–492. doi:10.18268/BSGM2016v68n3a6.
- McIlroy, Duncan; Green, O. R.; Brasier, M. D. (2001). "Palaeobiology and evolution of the earliest agglutinated Foraminifera: Platysolenites, Spirosolenites and related forms". Lethaia. 34 (1): 13–29. doi:10.1080/002411601300068170. ISSN 1502-3931.
- Pawlowski, Jan; Holzmann, Maria; Berney, Cédric; Fahrni, José; Gooday, Andrew J.; Cedhagen, Tomas; Habura, Andrea; Bowser, Samuel S. (30 September 2003). "The evolution of early Foraminifera". Proceedings of the National Academy of Sciences. 100 (20): 11494–11498. doi:10.1073/pnas.2035132100. ISSN 0027-8424. PMID 14504394.
- Cushman, Joseph A.; Ellisor, Alva C. (1 January 1945). "The Foraminiferal Fauna of the Anahuac Formation". Journal of Paleontology. 19 (6): 545–572. JSTOR 1299203.
- Boardman, R.S.; Cheetham, A.H.; Rowell, A.J. (1987). Fossil Invertebrates. Wiley. ISBN 978-0865423022.
- Zachos, J.C.; Pagani, M.; Sloan, L.; Thomas, E.; Billups, K. (2001). "Trends, Rhythms, and Aberrations in Global Climate, 65 Ma to Present". Science. 292 (5517): 686–693. Bibcode:2001Sci...292..686Z. doi:10.1126/science.1059412. PMID 11326091.
- Branson, Oscar; Redfern, Simon A.T.; Tyliszczak, Tolek; Sadekov, Aleksey; Langer, Gerald; Kimoto, Katsunori; Elderfield, Henry (December 2013). "The coordination of Mg in foraminiferal calcite". Earth and Planetary Science Letters. 383: 134–141. Bibcode:2013E&PSL.383..134B. doi:10.1016/j.epsl.2013.09.037.
- Misra, S.; Froelich, P. N. (26 January 2012). "Lithium Isotope History of Cenozoic Seawater: Changes in Silicate Weathering and Reverse Weathering". Science. 335 (6070): 818–823. Bibcode:2012Sci...335..818M. doi:10.1126/science.1214697. PMID 22282473.
- Hemming, N.G.; Hanson, G.N. (January 1992). "Boron isotopic composition and concentration in modern marine carbonates". Geochimica et Cosmochimica Acta. 56 (1): 537–543. Bibcode:1992GeCoA..56..537H. doi:10.1016/0016-7037(92)90151-8.
- Jones, R.W. (1996). Micropalaeontology in petroleum exploration. Clarendon Press. ISBN 978-0-19-854091-5.
- McNeil, D.H.; Issler, D.R.; Snowdon, L.R. (1996). Colour Alteration, Thermal Maturity, and Burial Diagenesis in Fossil Foraminifers. Geological Survey of Canada Bulletin. 499. Geological Survey of Canada. ISBN 978-0-660-16451-9.
External links
| Wikispecies has information related to Foraminifera |
| Wikimedia Commons has media related to Foraminifera. |
- General information
- The University of California Museum of Paleontology website has an Introduction to the Foraminifera
- Researchers at the University of South Florida developed a system using Foraminifera for monitoring coral reef environments
- University College London's micropaleontology site has an overview of Foraminifera, including many high-quality SEMs
- Illustrated glossary of terms used in foraminiferal research is the Lukas Hottinger's glossary published in the OA e-journal "Carnets de Géologie — Notebooks on Geology"
- Information on Foraminifera Martin Langer's Micropaleontology Page
- Benthic Foraminifera information from the 2005 Urbino Summer School of Paleoclimatology
- Online flip-books
- Illustrated glossary of terms used in foraminiferal research by Lukas Hottinger (alternative version of the one published in "Carnets de Géologie — Notebooks on Geology")
- Resources
- pforams@mikrotax - an online database detailing the taxonomy of planktonic foraminifera
- The star*sand project (part of micro*scope) is a cooperative database of information about Foraminifera
- 3D models of forams, generated by X-ray tomography
- CHRONOS has several Foraminifera resources, including a taxon search page and a micro-paleo section NB Most of this content is now included in the pforams@mikrotax website
- eForams is a web site focused on Foraminifera and modeling of foraminiferal shells
- Foraminifera Gallery Illustrated catalog of recent and fossil Foraminifera by genus and locality
- "Foraminifera". NCBI Taxonomy Browser. 29178.