Coulomb's law
Coulomb's law, or Coulomb's inverse-square law, is an experimental law[1] of physics that quantifies the amount of force between two stationary, electrically charged particles. The electric force between charged bodies at rest is conventionally called electrostatic force[2] or Coulomb force.[3] The quantity of electrostatic force between stationary charges is always described by Coulomb's law.[4] The law was first published in 1785 by French physicist Charles-Augustin de Coulomb, and was essential to the development of the theory of electromagnetism, maybe even its starting point,[5] because it was now possible to discuss quantity of electric charge in a meaningful way.[6]
| Part of a series of articles about |
| Electromagnetism |
|---|
 |
|
|
|
|
Covariant formulation
|
In its scalar form, the law is:
where ke is Coulomb's constant (ke ≈ 9×109 N⋅m2⋅C−2),[7] q1 and q2 are the signed magnitudes of the charges, and the scalar r is the distance between the charges. The force of the interaction between the charges is attractive if the charges have opposite signs (i.e., F is negative) and repulsive if like-signed (i.e., F is positive).
Being an inverse-square law, the law is analogous to Isaac Newton's inverse-square law of universal gravitation, but gravitational forces are always attractive, while electrostatic forces can be attractive or repulsive.[8] Coulomb's law can be used to derive Gauss's law, and vice versa. In the case of a single stationary point charge, the two laws are equivalent, expressing the same physical law in different ways.[9] The law has been tested extensively, and observations have upheld the law on a scale from 10−16 m to 108 m.[10]
History

Ancient cultures around the Mediterranean knew that certain objects, such as rods of amber, could be rubbed with cat's fur to attract light objects like feathers and papers. Thales of Miletus made a series of observations on static electricity around 600 BC, from which he believed that friction rendered amber magnetic, in contrast to minerals such as magnetite, which needed no rubbing.[11][12] Thales was incorrect in believing the attraction was due to a magnetic effect, but later science would prove a link between magnetism and electricity. Electricity would remain little more than an intellectual curiosity for millennia until 1600, when the English scientist William Gilbert made a careful study of electricity and magnetism, distinguishing the lodestone effect from static electricity produced by rubbing amber.[11] He coined the New Latin word electricus ("of amber" or "like amber", from ἤλεκτρον [elektron], the Greek word for "amber") to refer to the property of attracting small objects after being rubbed.[13] This association gave rise to the English words "electric" and "electricity", which made their first appearance in print in Thomas Browne's Pseudodoxia Epidemica of 1646.[14]
Early investigators of the 18th century who suspected that the electrical force diminished with distance as the force of gravity did (i.e., as the inverse square of the distance) included Daniel Bernoulli[15] and Alessandro Volta, both of whom measured the force between plates of a capacitor, and Franz Aepinus who supposed the inverse-square law in 1758.[16]
Based on experiments with electrically charged spheres, Joseph Priestley of England was among the first to propose that electrical force followed an inverse-square law, similar to Newton's law of universal gravitation. However, he did not generalize or elaborate on this.[17] In 1767, he conjectured that the force between charges varied as the inverse square of the distance.[18][19]
In 1769, Scottish physicist John Robison announced that, according to his measurements, the force of repulsion between two spheres with charges of the same sign varied as x−2.06.[20]
In the early 1770s, the dependence of the force between charged bodies upon both distance and charge had already been discovered, but not published, by Henry Cavendish of England.[21]
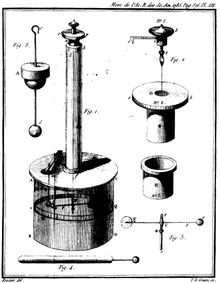
Finally, in 1785, the French physicist Charles-Augustin de Coulomb published his first three reports of electricity and magnetism where he stated his law. This publication was essential to the development of the theory of electromagnetism.[22] He used a torsion balance to study the repulsion and attraction forces of charged particles, and determined that the magnitude of the electric force between two point charges is directly proportional to the product of the charges and inversely proportional to the square of the distance between them.
The torsion balance consists of a bar suspended from its middle by a thin fiber. The fiber acts as a very weak torsion spring. In Coulomb's experiment, the torsion balance was an insulating rod with a metal-coated ball attached to one end, suspended by a silk thread. The ball was charged with a known charge of static electricity, and a second charged ball of the same polarity was brought near it. The two charged balls repelled one another, twisting the fiber through a certain angle, which could be read from a scale on the instrument. By knowing how much force it took to twist the fiber through a given angle, Coulomb was able to calculate the force between the balls and derive his inverse-square proportionality law.
The law
Coulomb's law states that:
The magnitude of the electrostatic force of attraction or repulsion between two point charges is directly proportional to the product of the magnitudes of charges and inversely proportional to the square of the distance between them.[22] The force is along the straight line joining them. If the two charges have the same sign, the electrostatic force between them is repulsive; if they have different signs, the force between them is attractive.

Coulomb's law can also be stated as a simple mathematical expression. The scalar and vector forms of the mathematical equation are
- and respectively,
where ke is Coulomb's constant (ke = 8.9875517873681764×109 N⋅m2⋅C−2), q1 and q2 are the signed magnitudes of the charges, the scalar r is the distance between the charges, the vector r21 = r1 − r2 is the vectorial distance between the charges, and r̂21 = r21/|r21| (a unit vector pointing from q2 to q1). The vector form of the equation calculates the force F1 applied on q1 by q2. If r12 is used instead, then the effect on q2 can be found. It can be also calculated using Newton's third law: F2 = −F1.
Units
When the electromagnetic theory is expressed in the International System of Units, force is measured in newtons, charge in coulombs, and distance in meters. Coulomb's constant is given by ke = 1/4πε0. The constant ε0 is the vacuum electric permittivity (also known as "electric constant") [23] in C2⋅m−2⋅N−1. It should not be confused with εr, which is the dimensionless relative permittivity of the material in which the charges are immersed, or with their product εa = ε0εr , which is called "absolute permittivity of the material" and is still used in electrical engineering.
The SI derived units for the electric field are volts per meter, newtons per coulomb, or tesla meters per second.
Coulomb's law and Coulomb's constant can also be interpreted in various terms:
- Atomic units. In atomic units the force is expressed in hartrees per Bohr radius, the charge in terms of the elementary charge, and the distances in terms of the Bohr radius.
- Electrostatic units or Gaussian units. In electrostatic units and Gaussian units, the unit charge (esu or statcoulomb) is defined in such a way that the Coulomb constant k disappears because it has the value of one and becomes dimensionless.
- Lorentz–Heaviside units (also called rationalized). In Lorentz–Heaviside units the Coulomb constant is ke = 1/4π and becomes dimensionless.
Gaussian units and Lorentz–Heaviside units are both CGS unit systems. Gaussian units are more amenable for microscopic problems such as the electrodynamics of individual electrically charged particles.[24] SI units are more convenient for practical, large-scale phenomena, such as engineering applications.[24]
Electric field
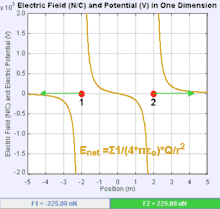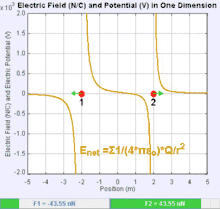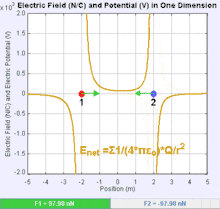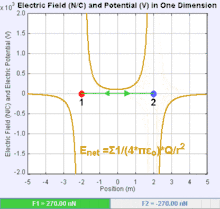
An electric field is a vector field that associates to each point in space the Coulomb force experienced by a test charge. In the simplest case, the field is considered to be generated solely by a single source point charge. The strength and direction of the Coulomb force F on a test charge qt depends on the electric field E that it finds itself in, such that F = qtE. If the field is generated by a positive source point charge q, the direction of the electric field points along lines directed radially outwards from it, i.e. in the direction that a positive point test charge qt would move if placed in the field. For a negative point source charge, the direction is radially inwards.
The magnitude of the electric field E can be derived from Coulomb's law. By choosing one of the point charges to be the source, and the other to be the test charge, it follows from Coulomb's law that the magnitude of the electric field E created by a single source point charge q at a certain distance from it r in vacuum is given by:
Coulomb's constant
Coulomb's constant is a proportionality factor that appears in Coulomb's law as well as in other electric-related formulas. The value of this constant is dependent upon the medium that the charged objects are immersed in. Denoted ke, it is also called the electric force constant or electrostatic constant,[8] hence the subscript e.
Prior to the 2019 redefinition of the SI base units, the Coulomb constant (in the case of air or vacuum) was considered to have an exact value:
Since the 2019 redefinition,[25][26] the Coulomb constant is no longer exactly defined and is subject to the measurement error in the fine structure constant. As calculated from CODATA 2018 recommended values, the Coulomb constant is[27]
Limitations
There are three conditions to be fulfilled for the validity of Coulomb's law:
- The charges must have a spherically symmetric distribution (e.g. be point charges, or a charged metal sphere).
- The charges must not overlap (e.g. they must be distinct point charges).
- The charges must be stationary with respect to each other.
The last of these is known as the electrostatic approximation. When movement takes place, Einstein's theory of relativity must be taken into consideration, and a result, an extra factor is introduced, which alters the force produced on the two objects. This extra part of the force is called the magnetic force, and is described by magnetic fields. For slow movement, the magnetic force is minimal and Coulomb's law can still be considered approximately correct, but when the charges are moving more quickly in relation to each other, the full electrodynamic rules (incorporating the magnetic force) must be considered.
Quantum field theory origin
In simple terms, the Coulomb potential derives from the QED Lagrangian as follows. The Lagrangian of quantum electrodynamics is normally written in natural units, but in SI units, it is:
where the covariant derivative (in SI units) is:
where is the gauge coupling parameter. By putting the covariant derivative into the lagrangian explicitly, the interaction term (the term involving both and ) is seen to be:
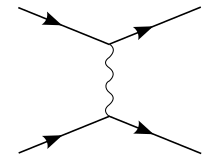
The most basic Feynman diagram for a QED interaction between two fermions is the exchange of a single photon, with no loops. Following the Feynman rules, this therefore contributes two QED vertex factors () to the potential, where Q is the QED-charge operator (Q gives the charge in terms of the electron charge, and hence is exactly −1 for electrons, etc.). For the photon in the diagram, the Feynman rules demand the contribution of one bosonic massless propagator . Ignoring the momentum on the external legs (the fermions), the potential is therefore:
which can be more usefully written as
where is the QED-charge on the ith particle. Recognising the integral as just being a Fourier transform enables the equation to be simplified:
For various reasons, it is more convenient to define the fine-structure constant , and then define . Rearranging these definitions gives:
In natural units (, , and ), . Continuing in SI units, the potential is therefore
Defining , as the macroscopic 'electric charge', makes e the macroscopic 'electric charge' for an electron, and enables the formula to be put into the familiar form of the Coulomb potential:
The force () is therefore :
The derivation makes clear that the force law is only an approximation — it ignores the momentum of the input and output fermion lines, and ignores all quantum corrections (i.e. the myriad possible diagrams with internal loops).
The Coulomb potential, and its derivation, can be seen as a special case of the Yukawa potential (specifically, the case where the exchanged boson – the photon – has no rest mass).
Scalar form

When it is of interest to know the magnitude of the electrostatic force (and not its direction), it may be easiest to consider a scalar version of the law. The scalar form of Coulomb's Law relates the magnitude and sign of the electrostatic force F acting simultaneously on two point charges q1 and q2 as follows:
where r is the separation distance and ke is Coulomb's constant. If the product q1q2 is positive, the force between the two charges is repulsive; if the product is negative, the force between them is attractive.[28]
Vector form

Coulomb's law states that the electrostatic force F1 experienced by a charge, q1 at position r1, in the vicinity of another charge, q2 at position r2, in a vacuum is equal to:
where r21 = r1 − r2, the unit vector r̂21 = r21/|r21|, and ε0 is the electric constant.
The vector form of Coulomb's law is simply the scalar definition of the law with the direction given by the unit vector, r̂21, parallel with the line from charge q2 to charge q1.[29] If both charges have the same sign (like charges) then the product q1q2 is positive and the direction of the force on q1 is given by r̂21; the charges repel each other. If the charges have opposite signs then the product q1q2 is negative and the direction of the force on q1 is given by −r̂21 = r̂12; the charges attract each other.
The electrostatic force F2 experienced by q2, according to Newton's third law, is F2 = −F1.
System of discrete charges
The law of superposition allows Coulomb's law to be extended to include any number of point charges. The force acting on a point charge due to a system of point charges is simply the vector addition of the individual forces acting alone on that point charge due to each one of the charges. The resulting force vector is parallel to the electric field vector at that point, with that point charge removed.
The force F on a small charge q at position r, due to a system of N discrete charges in vacuum is:
where qi and ri are the magnitude and position respectively of the ith charge, R̂i is a unit vector in the direction of Ri = r − ri (a vector pointing from charges qi to q).[29]
Continuous charge distribution
In this case, the principle of linear superposition is also used. For a continuous charge distribution, an integral over the region containing the charge is equivalent to an infinite summation, treating each infinitesimal element of space as a point charge dq. The distribution of charge is usually linear, surface or volumetric.
For a linear charge distribution (a good approximation for charge in a wire) where λ(r′) gives the charge per unit length at position r′, and dℓ′ is an infinitesimal element of length,
For a surface charge distribution (a good approximation for charge on a plate in a parallel plate capacitor) where σ(r′) gives the charge per unit area at position r′, and dA′ is an infinitesimal element of area,
For a volume charge distribution (such as charge within a bulk metal) where ρ(r′) gives the charge per unit volume at position r′, and dV′ is an infinitesimal element of volume,
The force on a small test charge q′ at position r in vacuum is given by the integral over the distribution of charge:
Simple experiment to verify Coulomb's law
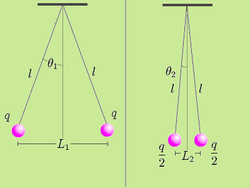
It is possible to verify Coulomb's law with a simple experiment. Consider two small spheres of mass m and same-sign charge q, hanging from two ropes of negligible mass of length l. The forces acting on each sphere are three: the weight mg, the rope tension T and the electric force F.
In the equilibrium state:
|
(1) |
and:
|
(2) |
|
(3) |
Let L1 be the distance between the charged spheres; the repulsion force between them F1, assuming Coulomb's law is correct, is equal to
|
(Coulomb's law) |
so:
|
(4) |
If we now discharge one of the spheres, and we put it in contact with the charged sphere, each one of them acquires a charge q/2. In the equilibrium state, the distance between the charges will be L2 < L1 and the repulsion force between them will be:
|
(5) |
We know that F2 = mg tan θ2. And:
|
(6) |
Measuring the angles θ1 and θ2 and the distance between the charges L1 and L2 is sufficient to verify that the equality is true taking into account the experimental error. In practice, angles can be difficult to measure, so if the length of the ropes is sufficiently great, the angles will be small enough to make the following approximation:
|
(7) |
Using this approximation, the relationship (6) becomes the much simpler expression:
|
(8) |
In this way, the verification is limited to measuring the distance between the charges and check that the division approximates the theoretical value.
Atomic forces
Coulomb's law holds even within atoms, correctly describing the force between the positively charged atomic nucleus and each of the negatively charged electrons. This simple law also correctly accounts for the forces that bind atoms together to form molecules and for the forces that bind atoms and molecules together to form solids and liquids. Generally, as the distance between ions increases, the force of attraction, and binding energy, approach zero and ionic bonding is less favorable. As the magnitude of opposing charges increases, energy increases and ionic bonding is more favorable.
Relation to Gauss's law
Deriving Gauss's law from Coulomb's law
Strictly speaking, Gauss's law cannot be derived from Coulomb's law alone, since Coulomb's law gives the electric field due to an individual point charge only. However, Gauss's law can be proven from Coulomb's law if it is assumed, in addition, that the electric field obeys the superposition principle. The superposition principle says that the resulting field is the vector sum of fields generated by each particle (or the integral, if the charges are distributed smoothly in space).
Outline of proof Coulomb's law states that the electric field due to a stationary point charge is: where
- er is the radial unit vector,
- r is the radius, |r|,
- ε0 is the electric constant,
- q is the charge of the particle, which is assumed to be located at the origin.
Using the expression from Coulomb's law, we get the total field at r by using an integral to sum the field at r due to the infinitesimal charge at each other point s in space, to give
where ρ is the charge density. If we take the divergence of both sides of this equation with respect to r, and use the known theorem[31]
where δ(r) is the Dirac delta function, the result is
Using the "sifting property" of the Dirac delta function, we arrive at
which is the differential form of Gauss' law, as desired.
Note that since Coulomb's law only applies to stationary charges, there is no reason to expect Gauss's law to hold for moving charges based on this derivation alone. In fact, Gauss's law does hold for moving charges, and in this respect Gauss's law is more general than Coulomb's law.
Deriving Coulomb's law from Gauss's law
Strictly speaking, Coulomb's law cannot be derived from Gauss's law alone, since Gauss's law does not give any information regarding the curl of E (see Helmholtz decomposition and Faraday's law). However, Coulomb's law can be proven from Gauss's law if it is assumed, in addition, that the electric field from a point charge is spherically symmetric (this assumption, like Coulomb's law itself, is exactly true if the charge is stationary, and approximately true if the charge is in motion).
Outline of proof Taking S in the integral form of Gauss' law to be a spherical surface of radius r, centered at the point charge Q, we have By the assumption of spherical symmetry, the integrand is a constant which can be taken out of the integral. The result is
where r̂ is a unit vector pointing radially away from the charge. Again by spherical symmetry, E points in the radial direction, and so we get
which is essentially equivalent to Coulomb's law. Thus the inverse-square law dependence of the electric field in Coulomb's law follows from Gauss' law.
See also
| Wikimedia Commons has media related to Coulomb's law. |
- Biot–Savart law
- Darwin Lagrangian
- Electromagnetic force
- Gauss's law
- Method of image charges
- Molecular modelling
- Newton's law of universal gravitation, which uses a similar structure, but for mass instead of charge
- Static forces and virtual-particle exchange
Notes
- Huray 2010, p. 57
- Walker, Halliday & Resnick 2014, p. 611
- Walker, Halliday & Resnick 2014, p. 609
- Jackson 1999, p. 24
- Huray 2010, p. 2
- Roller, Duane; Roller, D.H.D. (1954). The development of the concept of electric charge: Electricity from the Greeks to Coulomb. Cambridge, MA: Harvard University Press. p. 79.
- Huray 2010, p. 7
- Walker, Halliday & Resnick 2014, p. 614
- Purcell & Morin 2013, p. 25
- Purcell & Morin 2013, p. 11
- Stewart, Joseph (2001). Intermediate Electromagnetic Theory. World Scientific. p. 50. ISBN 978-981-02-4471-2.
- Simpson, Brian (2003). Electrical Stimulation and the Relief of Pain. Elsevier Health Sciences. pp. 6–7. ISBN 978-0-444-51258-1.
- Baigrie, Brian (2007). Electricity and Magnetism: A Historical Perspective. Greenwood Press. pp. 7–8. ISBN 978-0-313-33358-3.
- Chalmers, Gordon (1937). "The Lodestone and the Understanding of Matter in Seventeenth Century England". Philosophy of Science. 4 (1): 75–95. doi:10.1086/286445.
- Socin, Abel (1760). Acta Helvetica Physico-Mathematico-Anatomico-Botanico-Medica (in Latin). 4. Basileae. pp. 224–25.
- Heilbron, J.L. (1979). Electricity in the 17th and 18th Centuries: A Study of Early Modern Physics. Los Angeles, California: University of California Press. pp. 460–462 and 464 (including footnote 44). ISBN 978-0486406886.
- Schofield, Robert E. (1997). The Enlightenment of Joseph Priestley: A Study of his Life and Work from 1733 to 1773. University Park: Pennsylvania State University Press. pp. 144–56. ISBN 978-0-271-01662-7.
- Priestley, Joseph (1767). The History and Present State of Electricity, with Original Experiments. London, England. p. 732.
May we not infer from this experiment, that the attraction of electricity is subject to the same laws with that of gravitation, and is therefore according to the squares of the distances; since it is easily demonstrated, that were the earth in the form of a shell, a body in the inside of it would not be attracted to one side more than another?
- Elliott, Robert S. (1999). Electromagnetics: History, Theory, and Applications. ISBN 978-0-7803-5384-8.
- Robison, John (1822). Murray, John (ed.). A System of Mechanical Philosophy. 4. London, England.
On page 68, the author states that in 1769 he announced his findings regarding the force between spheres of like charge. On page 73, the author states the force between spheres of like charge varies as x−2.06:The result of the whole was, that the mutual repulsion of two spheres, electrified positively or negatively, was very nearly in the inverse proportion of the squares of the distances of their centres, or rather in a proportion somewhat greater, approaching to x−2.06.
When making experiments with charged spheres of opposite charge the results were similar, as stated on page 73:When the experiments were repeated with balls having opposite electricities, and which therefore attracted each other, the results were not altogether so regular and a few irregularities amounted to 1⁄6 of the whole; but these anomalies were as often on one side of the medium as on the other. This series of experiments gave a result which deviated as little as the former (or rather less) from the inverse duplicate ratio of the distances; but the deviation was in defect as the other was in excess.
Nonetheless, on page 74 the author infers that the actual action is related exactly to the inverse duplicate of the distance:We therefore think that it may be concluded, that the action between two spheres is exactly in the inverse duplicate ratio of the distance of their centres, and that this difference between the observed attractions and repulsions is owing to some unperceived cause in the form of the experiment.
On page 75, the authour compares the electric and gravitational forces:Therefore we may conclude, that the law of electric attraction and repulsion is similar to that of gravitation, and that each of those forces diminishes in the same proportion that the square of the distance between the particles increases.
- Maxwell, James Clerk, ed. (1967) [1879]. "Experiments on Electricity: Experimental determination of the law of electric force.". The Electrical Researches of the Honourable Henry Cavendish... (1st ed.). Cambridge, England: Cambridge University Press. pp. 104–113.
On pages 111 and 112 the author states:We may therefore conclude that the electric attraction and repulsion must be inversely as some power of the distance between that of the 2 + 1⁄50 th and that of the 2 − 1⁄50 th, and there is no reason to think that it differs at all from the inverse duplicate ratio.
- Coulomb (1785a) "Premier mémoire sur l’électricité et le magnétisme," Histoire de l’Académie Royale des Sciences, pp. 569–577 — Coulomb studied the repulsive force between bodies having electrical charges of the same sign:
Il résulte donc de ces trois essais, que l'action répulsive que les deux balles électrifées de la même nature d'électricité exercent l'une sur l'autre, suit la raison inverse du carré des distances. Translation: It follows therefore from these three tests, that the repulsive force that the two balls — [that were] electrified with the same kind of electricity — exert on each other, follows the inverse proportion of the square of the distance.
Coulomb also showed that oppositely charged bodies obey an inverse-square law of attraction.— Coulomb (1785b) "Second mémoire sur l’électricité et le magnétisme," Histoire de l’Académie Royale des Sciences, pages 578–611 - International Bureau of Weights and Measures (2019-05-20), SI Brochure: The International System of Units (SI) (PDF) (9th ed.), ISBN 978-92-822-2272-0, p. 15
- Jackson 1999, p. 784
- BIPM statement: Information for users about the proposed revision of the SI (PDF)
- "Decision CIPM/105-13 (October 2016)". The day is the 144th anniversary of the Metre Convention.
- Derived from ke = 1 / (4π ε0) – "2018 CODATA Value: vacuum electric permittivity". The NIST Reference on Constants, Units, and Uncertainty. NIST. 20 May 2019. Retrieved 2019-05-20.
- Coulomb's law, Hyperphysics
- Coulomb's law, University of Texas
- Charged rods, PhysicsLab.org
- See, for example, Griffiths, David J. (2013). Introduction to Electrodynamics (4th ed.). Prentice Hall. p. 50.
References
- Huray, Paul G. (2010). Maxwell's equations. Hoboken, NJ: Wiley. ISBN 0470542764.CS1 maint: ref=harv (link)
- Jackson, J. D. (1999) [1962]. Classical Electrodynamics (3rd ed.). New York: Wiley. ISBN 978-0-471-30932-1. OCLC 318176085.CS1 maint: ref=harv (link)
- Purcell, Edward M.; Morin, David J. (2013). Electricity and Magnetism (3rd ed.). Cambridge University Press. ISBN 9781107014022.CS1 maint: ref=harv (link)
- Walker, Jearl; Halliday, David; Resnick, Robert (2014). Fundamentals of physics (10th ed.). Hoboken, NJ: Wiley. ISBN 9781118230732. OCLC 950235056.CS1 maint: ref=harv (link)
Related reading
- Coulomb, Charles Augustin (1788) [1785]. "Premier mémoire sur l'électricité et le magnétisme". Histoire de l’Académie Royale des Sciences. Imprimerie Royale. pp. 569–577.
- Coulomb, Charles Augustin (1788) [1785]. "Second mémoire sur l'électricité et le magnétisme". Histoire de l’Académie Royale des Sciences. Imprimerie Royale. pp. 578–611.
- Coulomb, Charles Augustin (1788) [1785]. "Troisième mémoire sur l'électricité et le magnétisme". Histoire de l’Académie Royale des Sciences. Imprimerie Royale. pp. 612–638.
- Griffiths, David J. (1999). Introduction to Electrodynamics (3rd ed.). Prentice Hall. ISBN 978-0-13-805326-0.
- Tamm, Igor E. (1979) [1976]. Fundamentals of the Theory of Electricity (9th ed.). Moscow: Mir. pp. 23–27.
- Tipler, Paul A.; Mosca, Gene (2008). Physics for Scientists and Engineers (6th ed.). New York: W. H. Freeman and Company. ISBN 978-0-7167-8964-2. LCCN 2007010418.
- Young, Hugh D.; Freedman, Roger A. (2010). Sears and Zemansky's University Physics : With Modern Physics (13th ed.). Addison-Wesley (Pearson). ISBN 978-0-321-69686-1.
External links
- Coulomb's Law on Project PHYSNET
- Electricity and the Atom—a chapter from an online textbook
- A maze game for teaching Coulomb's Law—a game created by the Molecular Workbench software
- Electric Charges, Polarization, Electric Force, Coulomb's Law Walter Lewin, 8.02 Electricity and Magnetism, Spring 2002: Lecture 1 (video). MIT OpenCourseWare. License: Creative Commons Attribution-Noncommercial-Share Alike.