Deuterostome
Deuterostomes (taxonomic term: Deuterostomia; meaning "second mouth" in Greek)[1][2] constitute a superphylum of animals. It is a sister clade of Protostomia, with which it forms the Nephrozoa clade.
| Deuterostomes | |
|---|---|
 | |
| Examples of deuterostomes | |
| Scientific classification | |
| (unranked): | Filozoa |
| Kingdom: | Animalia |
| Subkingdom: | Eumetazoa |
| Clade: | ParaHoxozoa |
| Clade: | Bilateria |
| Clade: | Nephrozoa |
| Superphylum: | Deuterostomia Grobben, 1908 |
| Clades | |
Deuterostomia is a subtaxon of the Bilateria branch of the subkingdom Eumetazoa, within Animalia, and are distinguished from protostomes by their deuterostomic embryonic development; in deuterostomes, the first opening (the blastopore) becomes the anus, while in protostomes, it becomes the mouth. (There are some occurrences of deuterostomy among protostomes.)[3]
Deuterostomes are also known as enterocoelomates because their coelom develops through enterocoely.
There are three major clades of deuterostomes:
- Chordata (vertebrates and their kin)
- Echinodermata (starfish, sea urchins, sea cucumbers)
- Hemichordata (acorn worms and graptolites)
Systematics
History
Initially, Deuterostomia included the phyla Brachiopoda,[4] Bryozoa,[5] Chaetognatha,[6] and Phoronida[4] based on morphological and embryological characteristics. However, Superphylum Deuterostomia was redefined in 1995 based on DNA molecular sequence analyses when the lophophorates were removed from it and combined with other protostome animals to form superphylum Lophotrochozoa.[7] The phylum Chaetognatha (arrow worms) may belong here,[6] but molecular studies have placed them in the protostomes more often.[8][9]
Extinct deuterostome groups may include the phylum Vetulicolia.
Classification
These are the following phyla/subgroups of the deuterostomes:
- Superphylum Deuterostomia
- Phylum Chordata (vertebrates, tunicates, and lancelets)
- Subphylum Cephalochordata – 1 class (lancelets)
- Subphylum Tunicata (Urochordata) – 4 classes (tunicates)
- Subphylum Vertebrata (Craniata) – 9 classes (vertebrates – mammals, reptiles, amphibians, birds, and fish)
- Infraphylum Agnatha (Cyclostomata or incertae sedis) – 2 classes (jawless fish – hagfish and lampreys)
- Infraphylum Gnathostomata – 7 classes (jawed vertebrates – mammals, reptiles, amphibians, birds, bony fish, and cartilaginous fish)
- Superclass incertae sedis – 1 class (cartilaginous fish – sharks, skates, rays, and chimaeras)
- Superclass Osteichthyes – 2 classes (bony fish, 98.8 percent of all fish – ray-finned fish and lobe-finned fish)
- Superclass Tetrapoda – 4 classes (four-limbed vertebrates – mammals, reptiles, amphibians, and birds)
- Phylum Hemichordata – 3 classes (hemichordates, known as acorn worms)
- Phylum Echinodermata (echinoderms – sea stars, brittle stars, sea lilies, sea urchins, and sea cucumbers)
- Subphylum Asterozoa – 2 classes (sea stars and brittle stars)
- Subphylum Crinozoa – 1 class (sea lilies)
- Subphylum Echinozoa – 2 classes (sea urchins and sea cucumbers)
- Phylum Chordata (vertebrates, tunicates, and lancelets)
Echinodermata and Hemichordata form the clade Ambulacraria. Moreover, there is a possibility that Ambulacraria can be the sister clade to Xenacoelomorpha, and form the Xenambulacraria group.[10][11][12]
Notable characteristics
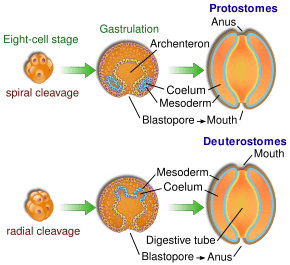
In both deuterostomes and protostomes, a zygote first develops into a hollow ball of cells, called a blastula. In deuterostomes, the early divisions occur parallel or perpendicular to the polar axis. This is called radial cleavage, and also occurs in certain protostomes, such as the lophophorates.
Most deuterostomes display indeterminate cleavage, in which the developmental fate of the cells in the developing embryo are not determined by the identity of the parent cell. Thus, if the first four cells are separated, each cell is capable of forming a complete small larva; and if a cell is removed from the blastula, the other cells will compensate.
In deuterostomes the mesoderm forms as evaginations of the developed gut that pinch off, forming the coelom. This is called enterocoely.
Another feature present in both the Hemichordata and Chordata is pharyngotremy; the presence of spiracles or gill slits into the pharynx, which is also found in some primitive fossil echinoderms (mitrates).[13][14] A hollow nerve cord is found in all chordates, including tunicates (in the larval stage). Some hemichordates also have a tubular nerve cord. In the early embryonic stage, it looks like the hollow nerve cord of chordates.
Because of the highly modified nervous system of echinoderms, it is not possible to discern much about their ancestors in this matter, but based on different facts it is quite possible that all the present deuterostomes evolved from a common ancestor that had pharyngeal gill slits, a hollow nerve cord, circular and longitudinal muscles and a segmented body.[15] It could have resembled the small group of Cambrian urochordate deuterostomes named Vetulicolia.
Formation of mouth and anus
The defining characteristic of the deuterostome is the fact that the blastopore (the opening at the bottom of the forming gastrula) becomes the anus, whereas in protostomes the blastopore becomes the mouth. The deuterostome mouth develops at the opposite end of the embryo from the blastopore and a digestive tract develops in the middle, connecting the two.
In many animals these early development stages later evolved in ways that no longer reflect these original patterns. For instance, humans have already formed a gut tube at the time of formation of the mouth and anus. Then the mouth forms first, during the fourth week of development, and the anus forms four weeks later, temporarily forming a cloaca.
Origins and evolution
The majority of animals more complex than jellyfish and other Cnidarians are split into two groups, the protostomes and deuterostomes. Chordates (which include all the vertebrates) are deuterostomes.[16] It seems likely that the 555 million year old Kimberella was a member of the protostomes.[17][18] That implies that the protostome and deuterostome lineages split some time before Kimberella appeared — at least 558 million years ago, and hence well before the start of the Cambrian 541 million years ago,[16] i.e. during the later part of the Ediacaran Period (circa 635-542 Mya, around the end of global Marinoan glaciation in the late Neoproterozoic). The oldest discovered proposed deuterostome is Saccorhytus coronarius, which lived approximately 540 million years ago.[2][19] The researchers that made the discovery believe that the Saccorhytus is a common ancestor to all previously-known deuterostomes.[19]
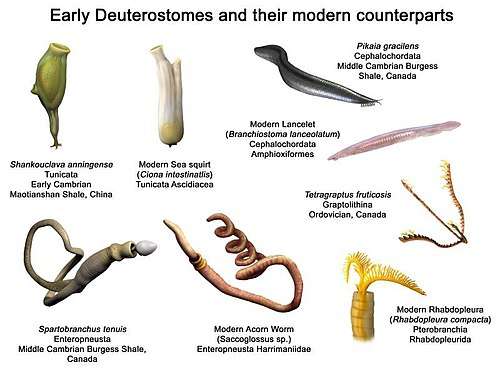
Fossils of one major deuterostome group, the echinoderms (whose modern members include sea stars, sea urchins and crinoids), are quite common from the start of Series 2 of the Cambrian, 521 million years ago.[20] The Mid Cambrian fossil Rhabdotubus johanssoni has been interpreted as a pterobranch hemichordate.[21] Opinions differ about whether the Chengjiang fauna fossil Yunnanozoon, from the earlier Cambrian, was a hemichordate or chordate.[22][23] Another Chengjiang fossil, Haikouella lanceolata, also from the Chengjiang fauna, is interpreted as a chordate and possibly a craniate, as it shows signs of a heart, arteries, gill filaments, a tail, a neural chord with a brain at the front end, and possibly eyes — although it also had short tentacles round its mouth.[23] Haikouichthys and Myllokunmingia, also from the Chengjiang fauna, are regarded as fish.[24][25] Pikaia, discovered much earlier but from the Mid Cambrian Burgess Shale, is also regarded as a primitive chordate.[26]
On the other hand, fossils of early chordates are very rare, as non-vertebrate chordates have no bone tissue or teeth, and fossils of no Post-Cambrian non-vertebrate chordates are known aside from the Permian-aged Paleobranchiostoma, trace fossils of the Ordovician colonial tunicate Catellocaula, and various Jurassic-aged and Tertiary-aged spicules tentatively attributed to ascidians.
Phylogeny
Below is a phylogenetic tree showing consensus relationships among deuterostome taxa. Phylogenomic evidence suggests the enteropneust family, Torquaratoridae, fall within the Ptychoderidae. The tree is based on 16S +18S rRNA sequence data and phylogenomic studies from multiple sources.[27] The approximate dates for each radiation into a new clade are given in millions of years ago (Mya). Not all dates are consistent, as of date ranges only the center is given.[28]
| Nephrozoa |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 575 mya |
See also
- Timeline of the evolutionary history of life – Current scientific theory outlining the major events during the development of life
References
- Wade, Nicholas (30 January 2017). "This Prehistoric Human Ancestor Was All Mouth". The New York Times. Retrieved 31 January 2017.
- Han, Jian; Morris, Simon Conway; Ou, Qiang; Shu, Degan; Huang, Hai (2017). "Meiofaunal deuterostomes from the basal Cambrian of Shaanxi (China)". Nature. 542 (7640): 228–231. Bibcode:2017Natur.542..228H. doi:10.1038/nature21072. ISSN 0028-0836. PMID 28135722.
- Martín-Durán, José M.; Passamaneck, Yale J.; Martindale, Mark Q.; Hejnol, Andreas (2016). "The developmental basis for the recurrent evolution of deuterostomy and protostomy". Nature Ecology & Evolution. 1 (1): 0005. doi:10.1038/s41559-016-0005. PMID 28812551.
- Eernisse, Douglas J.; Albert, James S.; Anderson, Frank E. (1992-09-01). "Annelida and Arthropoda are Not Sister Taxa: A Phylogenetic Analysis of Spiralian Metazoan Morphology". Systematic Biology. 41 (3): 305–330. doi:10.1093/sysbio/41.3.305. ISSN 1063-5157.
- Nielsen, C. (July 2002). "The Phylogenetic Position of Entoprocta, Ectoprocta, Phoronida, and Brachiopoda". Integrative and Comparative Biology. 42 (3): 685–691. doi:10.1093/icb/42.3.685. PMID 21708765.
- Brusca, R.C.; Brusca, G.J. (1990). Invertebrates. Sinauer Associates. p. 669.
- Halanych, K.M.; Bacheller, J.; Liva, S.; Aguinaldo, A. A.; Hillis, D.M. & Lake, J.A. (17 March 1995). "18S rDNA evidence that the Lophophorates are Protostome Animals". Science. 267 (5204): 1641–1643. Bibcode:1995Sci...267.1641H. doi:10.1126/science.7886451. PMID 7886451.
- Marlétaz, Ferdinand; Martin, Elise; Perez, Yvan; Papillon, Daniel; Caubit, Xavier; Lowe, Christopher J.; Freeman, Bob; Fasano, Laurent; Dossat, Carole; Wincker, Patrick; Weissenbach, Jean (2006-08-01). "Chaetognath phylogenomics: a protostome with deuterostome-like development". Current Biology. 16 (15): R577–R578. doi:10.1016/j.cub.2006.07.016. PMID 16890510.
- Marlétaz, Ferdinand; Peijnenburg, Katja T.C.A.; Goto, Taichiro; Satoh, Noriyuki; Rokhsar, Daniel S. (2019-01-21). "A New Spiralian Phylogeny Places the Enigmatic Arrow Worms among Gnathiferans". Current Biology. 29 (2): 312–318.e3. doi:10.1016/j.cub.2018.11.042. PMID 30639106.
- Bourlat, Sarah J.; Juliusdottir, Thorhildur; Lowe, Christopher J.; Freeman, Robert; Aronowicz, Jochanan; Kirschner, Mark; Lander, Eric S.; Thorndyke, Michael; Nakano, Hiroaki; Kohn, Andrea B.; Heyland, Andreas; Moroz, Leonid L.; Copley, Richard R.; Telford, Maximilian J. (2006). "Deuterostome phylogeny reveals monophyletic chordates and the new phylum Xenoturbellida". Nature. 444 (7115): 85–88. Bibcode:2006Natur.444...85B. doi:10.1038/nature05241. ISSN 0028-0836. PMID 17051155.
- Philippe, Hervé; Poustka, Albert J.; Chiodin, Marta; Hoff, Katharina J.; Dessimoz, Christophe; Tomiczek, Bartlomiej; Schiffer, Philipp H.; Müller, Steven; Domman, Daryl; Horn, Matthias; Kuhl, Heiner; Timmermann, Bernd; Satoh, Noriyuki; Hikosaka-Katayama, Tomoe; Nakano, Hiroaki; Rowe, Matthew L.; Elphick, Maurice R.; Thomas-Chollier, Morgane; Hankeln, Thomas; Mertes, Florian; Wallberg, Andreas; Rast, Jonathan P.; Copley, Richard R.; Martinez, Pedro; Telford, Maximilian J. (2019). "Mitigating Anticipated Effects of Systematic Errors Supports Sister-Group Relationship between Xenacoelomorpha and Ambulacraria". Current Biology. 29 (11): 1818–1826.e6. doi:10.1016/j.cub.2019.04.009. hdl:21.11116/0000-0004-DC4B-1. ISSN 0960-9822. PMID 31104936.
- Marlétaz, Ferdinand (2019-06-17). "Zoology: Worming into the Origin of Bilaterians". Current Biology. 29 (12): R577–R579. doi:10.1016/j.cub.2019.05.006. ISSN 0960-9822. PMID 31211978.
- Graham, A; Richardson, J (2012). "Developmental and evolutionary origins of the pharyngeal apparatus". Evodevo. 3 (1): 24. doi:10.1186/2041-9139-3-24. PMC 3564725. PMID 23020903.
- On the Origin of Phyla
- Smith, Andrew B. (2012). "Cambrian problematica and the diversification of deuterostomes". BMC Biology. 10 (79): 79. doi:10.1186/1741-7007-10-79. PMC 3462677. PMID 23031503.
- Erwin, Douglas H.; Eric H. Davidson (1 July 2002). "The last common bilaterian ancestor". Development. 129 (13): 3021–3032. PMID 12070079.
- New data on Kimberella, the Vendian mollusc-like organism (White sea region, Russia): palaeoecological and evolutionary implications (2007), "Fedonkin, M.A.; Simonetta, A; Ivantsov, A.Y.", in Vickers-Rich, Patricia; Komarower, Patricia (eds.), The Rise and Fall of the Ediacaran Biota, Special publications, 286, London: Geological Society, pp. 157–179, doi:10.1144/SP286.12, ISBN 9781862392335, OCLC 156823511CS1 maint: uses authors parameter (link)
- Butterfield, N.J. (December 2006). "Hooking some stem-group "worms": fossil lophotrochozoans in the Burgess Shale". BioEssays. 28 (12): 1161–1166. doi:10.1002/bies.20507. PMID 17120226.
- Ghosh, Pallab (30 January 2017). "Scientists find 'oldest human ancestor'". BBC. Retrieved 30 January 2017.
- Bengtson, S. (2004). Lipps, J.H.; Waggoner, B.M. (eds.). "Early Skeletal Fossils in Neoproterozoic–Cambrian Biological Revolutions" (PDF). Paleontological Society Papers. 10: 67–78. doi:10.1017/S1089332600002345.
- Bengtson, S.; Urbanek, A. (October 2007). "Rhabdotubus, a Middle Cambrian rhabdopleurid hemichordate". Lethaia. 19 (4): 293–308. doi:10.1111/j.1502-3931.1986.tb00743.x.
- Shu, D.; Zhang, X. & Chen, L. (April 1996). "Reinterpretation of Yunnanozoon as the earliest known hemichordate". Nature. 380 (6573): 428–430. Bibcode:1996Natur.380..428S. doi:10.1038/380428a0.
- Chen, J-Y.; Hang, D-Y. & Li, C.W. (December 1999). "An early Cambrian craniate-like chordate". Nature. 402 (6761): 518–522. Bibcode:1999Natur.402..518C. doi:10.1038/990080.
- Shu, D-G.; Conway Morris, S.; Han, J.; et al. (January 2003). "Head and backbone of the Early Cambrian vertebrate Haikouichthys". Nature. 421 (6922): 526–529. Bibcode:2003Natur.421..526S. doi:10.1038/nature01264. PMID 12556891.
- Shu, D-G.; Conway Morris, S. & Zhang, X-L. (November 1999). "Lower Cambrian vertebrates from south China". Nature. 402 (6757): 42–46. Bibcode:1999Natur.402...42S. doi:10.1038/46965.
- Shu, D-G.; Conway Morris, S. & Zhang, X-L. (November 1996). "A Pikaia-like chordate from the Lower Cambrian of China". Nature. 384 (6605): 157–158. Bibcode:1996Natur.384..157S. doi:10.1038/384157a0.
- Tassia, Michael G.; Cannon, Johanna T.; Konikoff, Charlotte E.; Shenkar, Noa; Halanych, Kenneth M.; Swalla, Billie J. (2016-10-04). "The Global Diversity of Hemichordata". PLOS One. 11 (10): e0162564. Bibcode:2016PLoSO..1162564T. doi:10.1371/journal.pone.0162564. PMC 5049775. PMID 27701429.
- Han, Jian; Morris, Simon Conway; Ou, Qian; Shu, Degan; Huang, Hai (2017). "Meiofaunal deuterostomes from the basal Cambrian of Shaanxi (China)". Nature. 542 (7640): 228–231. Bibcode:2017Natur.542..228H. doi:10.1038/nature21072. PMID 28135722.
External links
| Wikispecies has information related to Deuterostomia |
| Wikimedia Commons has media related to Deuterostomia. |

