Colistin
Colistin, also known as polymyxin E, is an antibiotic used as a last-resort for multidrug-resistant Gram negative infections including pneumonia.[1][2] These may involve bacteria such as Pseudomonas aeruginosa, Klebsiella pneumoniae, or Acinetobacter.[3] It comes in a form which can be injection into a vein or muscle or inhaled, known as colistimethate sodium and one which is applied to the skin or taken by mouth, known as colistin sulfate.[4] Resistance to colistin is beginning to appear as of 2017.[5]
 | |
| Clinical data | |
|---|---|
| Trade names | Xylistin, Coly Mycin M, others |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | Topical, by mouth, intravenous, inhaled |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 0% |
| Elimination half-life | 5 hours |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.012.644 |
| Chemical and physical data | |
| Formula | C52H98N16O13 |
| Molar mass | 1155.4495 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
InChI
| |
| | |
Common side effects of the injectable form include kidney and neurological problems.[2] Other serious side effects may include anaphylaxis, muscle weakness, and Clostridium difficile-associated diarrhea.[2] The inhaled form may result in constriction of the bronchioles.[2] It is unclear if use during pregnancy is safe for the baby.[6] Colistin is in the polymyxin class of medications.[2] It works by breaking down the cytoplasmic membrane which generally results in bacterial cell death.[2]
Colistin was discovered in 1947 and colistimethate sodium was approved for medical use in the United States in 1970.[3][2] It is on the World Health Organization's List of Essential Medicines, the safest and most effective medicines needed in a health system.[7] It is available as a generic medication.[8] In the United Kingdom it costs the NHS about £5.40 per day for the injectable form as of 2018.[8] In the United States this amount costs about US$47 as of 2019.[9] It is derived from the bacterium Bacillus colistinus.[4]
Medical uses
Antibacterial spectrum
Colistin has been effective in treating infections caused by Pseudomonas, Escherichia, and Klebsiella species. The following represents MIC susceptibility data for a few medically significant microorganisms:[10][11]
- Escherichia coli: 0.12–128 μg/ml
- Klebsiella pneumoniae: 0.25–128 μg/ml
- Pseudomonas aeruginosa: ≤0.06–16 μg/ml
For example, colistin in combination with other drugs are used to attack P. aeruginosa biofilm infection in lungs of CF patients.[12] Biofilms have a low oxygen environment below the surface where bacteria are metabolically inactive and colistin is highly effective in this environment. However, P. aeruginosa reside in the top layers of the biofilm, where they remain metabolically active.[13] This is because surviving tolerant cells migrate to the top of the biofilm via pili motility and form new aggregates via quorum sensing.[14]
Administration and dosage
Forms
Two forms of colistin are available commercially: colistin sulfate and colistimethate sodium (colistin methanesulfonate sodium, colistin sulfomethate sodium). Colistin sulfate is cationic; colistimethate sodium is anionic. Colistin sulfate is stable, but colistimethate sodium is readily hydrolysed to a variety of methanesulfonated derivatives. Colistin sulfate and colistimethate sodium are eliminated from the body by different routes. With respect to Pseudomonas aeruginosa, colistimethate is the inactive prodrug of colistin. The two drugs are not interchangeable .
- Colistimethate sodium may be used to treat Pseudomonas aeruginosa infections in cystic fibrosis patients, and it has come into recent use for treating multidrug-resistant Acinetobacter infection, although resistant forms have been reported.[15][16] Colistimethate sodium has also been given intrathecally and intraventricularly in Acinetobacter baumannii and Pseudomonas aeruginosa meningitis/ventriculitis[17][18][19][20] Some studies have indicated that colistin may be useful for treating infections caused by carbapenem-resistant isolates of Acinetobacter baumannii.[16]
- Colistin sulfate may be used to treat intestinal infections, or to suppress colonic flora. Colistin sulfate is also used as topical creams, powders, and otic solutions.
- Colistin A (polymyxin E1) and colistin B (polymyxin E2) can be purified individually to research and study their effects and potencies as separate compounds.
Dosage
Colistin sulfate and colistimethate sodium may both be given intravenously, but the dosing is complicated. The very different labeling of the parenteral products of colistin methanesulfonate in different parts of the world was first revealed by Li et al.[21] Colistimethate sodium manufactured by Xellia (Colomycin injection) is prescribed in international units, but colistimethate sodium manufactured by Parkdale Pharmaceuticals (Coly-Mycin M Parenteral) is prescribed in milligrams of colistin base:
- Colomycin 1,000,000 units is 80 mg colistimethate;[22]
- Coly-mycin M 150 mg "colistin base" is 360 mg colistimethate or 4,500,000 units.[23]
Because colistin was introduced into clinical practice over 50 years ago, it was never subject to the regulations that modern drugs are subject to, and therefore there is no standardised dosing of colistin and no detailed trials on pharmacology or pharmacokinetics: The optimal dosing of colistin for most infections is therefore unknown. Colomycin has a recommended intravenous dose of 1 to 2 million units three times daily for patients weighing 60 kg or more with normal renal function. Coly-Mycin has a recommended dose of 2.5 to 5 mg/kg colistin base a day, which is equivalent to 6 to 12 mg/kg colistimethate sodium per day. For a 60 kg man, therefore, the recommended dose for Colomycin is 240 to 480 mg of colistimethate sodium, yet the recommended dose for Coly-Mycin is 360 to 720 mg of colistimethate sodium. Likewise, the recommended "maximum" dose for each preparation is different (480 mg for Colomycin and 720 mg for Coly-Mycin). Each country has different generic preparations of colistin, and the recommended dose depends on the manufacturer. This complete absence of any regulation or standardisation of dose makes intravenous colistin dosing difficult for any physician.
Colistin has been used in combination with rifampicin, and evidence of in-vitro synergy exists,[24][25] and the combination has been used successfully in patients.[26] There is also in-vitro evidence of synergy for colistimethate sodium used in combination with other antipseudomonal antibiotics.[27]
Colistimethate sodium aerosol (Promixin; Colomycin Injection) is used to treat pulmonary infections, especially in cystic fibrosis. In the UK, the recommended adult dose is 1–2 million units (80–160 mg) nebulised colistimethate twice daily.[28][22] Nebulized colistin has also been used to decrease severe exacerbations in patients with chronic obstructive pulmonary disease and infection with Pseudomonas aeruginosa.[29]
Resistance
Resistance to colistin is rare, but has been described. The first colistin-resistance gene in a plasmid which can be transferred between bacterial strains was found in 2011 in China and became publicly known in November 2015. The presence of this plasmid-borne mcr-1 gene was confirmed starting December 2015 in South-East Asia, several European countries and the United States. It is produced by certain strains of the bacteria Paenibacillus polymyxa.
As of 2017, no agreement exists about how to look for colistin resistance. The Société Française de Microbiologie uses a cut-off of 2 mg/l, whereas the British Society for Antimicrobial Chemotherapy sets a cutoff of 4 mg/l or less as sensitive, and 8 mg/ml or more as resistant. No standards for measuring colistin sensitivity are given in the US.
The plasmid-borne mcr-1 gene has been found to confer resistance to colistin.[30] The first colistin-resistance gene in a plasmid which can be transferred between bacterial strains was found in 2011 and became publicly known in November 2015.[30][31] This plasmid-borne mcr-1 gene has since been isolated in China,[30] Europe[32] and the United States.[33]
India reported the first detailed colistin-resistance study which mapped 13 colistin-resistant cases recorded over 18 months. It concluded that pan-drug resistant infections, particularly those in the blood stream, have a higher mortality. Multiple other cases were reported from other Indian hospitals.[34][35] Although resistance to polymyxins is generally less than 10%, it is more frequent in the Mediterranean and South-East Asia (Korea and Singapore), where colistin resistance rates are continually increasing.[36] Colistin-resistant E. coli was identified in the United States in May 2016.[37]
Use of colistin to treat Acinetobacter baumannii infections has led to the development of resistant bacterial strains. which have also developed resistance to antimicrobial compounds LL-37 and lysozyme, produced by the human immune system.[38]
It's worth noting that not all resistance to colisin and some other antibiotics is due to the presence of resistance genes.[39] Heteroresistance, the phenomenon wherein apparently genetically identical microbes exhibit a range of resistance to an antibiotic,[40] has been observed in some species of Enterobacter since at least 2016[39] and in some strains of Klebsiella pneumoniae in 2017-2018.[41] In some cases this phenomenon has significant clinical consequences.[41]
Inherently resistant
- Brucella
- Burkholderia cepacia
- Chryseobacterium indologenes
- Edwardsiella
- Elizabethkingia meningoseptica
- Francisella tularensis spp.
- Gram-negative cocci
- Helicobacter pylori
- Moraxella catarrhalis
- Morganella spp.
- Neisseria gonorrhoeae and Neisseria meningitidis
- Proteus
- Providencia
- Serratia
- Some strains of Stenotrophomonas maltophilia[42]
Variable resistance
- Aeromonas
- Vibrio
- Prevotella
- Fusobacterium
- Escherichia coli
Adverse reactions
The main toxicities described with intravenous treatment are nephrotoxicity (damage to the kidneys) and neurotoxicity (damage to the nerves),[43][44][45][46] but this may reflect the very high doses given, which are much higher than the doses currently recommended by any manufacturer and for which no adjustment was made for renal disease. Neuro- and nephrotoxic effects appear to be transient and subside on discontinuation of therapy or reduction in dose.[47]
At a dose of 160 mg colistimethate IV every eight hours, very little nephrotoxicity is seen.[48][49] Indeed, colistin appears to have less toxicity than the aminoglycosides that subsequently replaced it, and it has been used for extended periods up to six months with no ill effects.[50] The colistin-induced nephrotoxicity is particularly favoured in patients with hypoalbuminemia.[51]
The main toxicity described with aerosolised treatment is bronchospasm,[52] which can be treated or prevented with the use of beta2-agonists such as salbutamol[53] or following a desensitisation protocol.[54]
Mechanism of action
Colistin is a polycationic peptide and has both hydrophilic and lipophilic moieties. These cationic regions interact with the bacterial outer membrane, by displacing magnesium and calcium bacterial counter ions in the lipopolysaccharide. Hydrophobic/hydrophilic regions interact with the cytoplasmic membrane just like a detergent, solubilizing the membrane in an aqueous environment. This effect is bactericidal even in an isosmolar environment.
Pharmacokinetics
No clinically useful absorption of colistin occurs in the gastrointestinal tract. For systemic infection, colistin must, therefore, be given by injection. Colistimethate is eliminated by the kidneys, but colistin is supposed to be eliminated by non-renal mechanism(s) that are as of yet not characterised.[55][56]
History
Colistin was first isolated in Japan in 1949 from a flask of fermenting Bacillus polymyxa var. colistinus by the Japanese scientist Koyama[57] and became available for clinical use in 1959.[58]
Colistimethate sodium, a less toxic prodrug, became available for injection in 1959. In the 1980s, polymyxin use was widely discontinued because of nephro- and neurotoxicity. As multi-drug resistant bacteria became more prevalent in the 1990s, colistin started to get a second look as an emergency solution, in spite of toxic effects.[59]
Biosynthesis
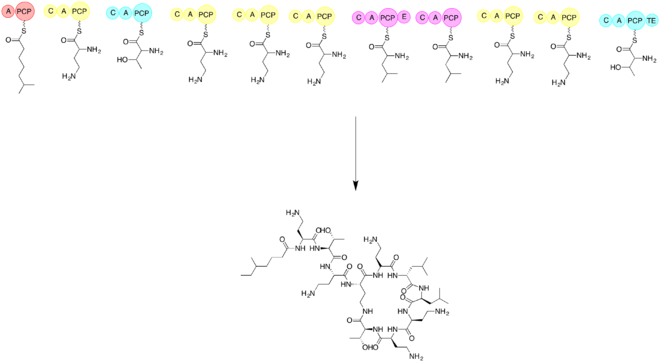
The biosynthesis of colistin requires the use of three amino acids threonine, leucine, and 2,4-diaminobutryic acid. It is important to synthesis the linear form of colistin before cycliziation. Elongation of non ribosomal peptide biosynthesis begins by a loading module and then the addition of each subsequent amino acid. The subsequent amino acids are added with the help of an adenylation domain (A), a peptidyl carrier protein domain (PCP), an epimerization domain (E), and a condensation domain (C). Cyclization is accomplished by utilizing a thioesterase.[60] The first step is to have a loading domain, 6-methyl-heptanoic acid, associate with the A and PCP domains. Now with a C, A, and PCP domain that is associated with 2,4-diaminobutryic acid. This continues with each amino acid until the linear peptide chain is completed. The last module will have a thioesterase to complete the cyclization and form the product colistin.
See also
- Drug of last resort
- Salvage therapy
References
- Pogue, JM; Ortwine, JK; Kaye, KS (April 2017). "Clinical considerations for optimal use of the polymyxins: A focus on agent selection and dosing". Clinical Microbiology and Infection. 23 (4): 229–233. doi:10.1016/j.cmi.2017.02.023. PMID 28238870.
- "Colistimethate Sodium Monograph for Professionals". Drugs.com. Retrieved 6 November 2019.
- Falagas ME, Grammatikos AP, Michalopoulos A (October 2008). "Potential of old-generation antibiotics to address current need for new antibiotics". Expert Review of Anti-infective Therapy. 6 (5): 593–600. doi:10.1586/14787210.6.5.593. PMID 18847400.
- Bennett, John E.; Dolin, Raphael; Blaser, Martin J.; Mandell, Gerald L. (2009). Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases E-Book. Elsevier Health Sciences. p. 469. ISBN 9781437720600.
- Caniaux, I; van Belkum, A; Zambardi, G; Poirel, L; Gros, MF (March 2017). "MCR: modern colistin resistance" (PDF). European Journal of Clinical Microbiology & Infectious Diseases. 36 (3): 415–420. doi:10.1007/s10096-016-2846-y. PMID 27873028.
- "Colistimethate (Coly Mycin M) Use During Pregnancy". Drugs.com. Retrieved 11 November 2019.
- Organization, World Health (2019). "World Health Organization model list of essential medicines: 21st list 2019". hdl:10665/325771. Cite journal requires
|journal=(help) - British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 547. ISBN 9780857113382.
- "Colistimethate Prices, Coupons & Patient Assistance Programs". Drugs.com. Retrieved 11 November 2019.
- "Polymyxin E (Colistin) - The Antimicrobial Index Knowledgebase - TOKU-E". Archived from the original on 28 May 2016. Retrieved 28 May 2016.
- "Archived copy" (PDF). Archived (PDF) from the original on 2016-03-04. Retrieved 2014-02-10.CS1 maint: archived copy as title (link)
- Herrmann G, Yang L, Wu H, Song Z, Wang H, Høiby N, Ulrich M, Molin S, Riethmüller J, Döring G (2010). "Colistin-tobramycin combinations are superior to monotherapy concerning the killing of biofilm Pseudomonas aeruginosa". J. Infect. Dis. 202 (10): 1585–92. doi:10.1086/656788. PMID 20942647.
- Pamp SJ, Gjermansen M, Johansen HK, Tolker-Nielsen T (2008). "Tolerance to the antimicrobial peptide colistin in Pseudomonas aeruginosa biofilms is linked to metabolically active cells, and depends on the pmr and mexAB-oprM genes". Molecular Microbiology. 68 (1): 223–240. doi:10.1111/j.1365-2958.2008.06152.x. PMID 18312276.
- Chua SL, Yam JK, Sze KS, Yang L (2016). "Selective labelling and eradication of antibiotic-tolerant bacterial populations in Pseudomonas aeruginosa biofilms". Nat Commun. 7: 10750. Bibcode:2016NatCo...710750C. doi:10.1038/ncomms10750. PMC 4762895. PMID 26892159.
- Reis AO, Luz DA, Tognim MC, Sader HS, Gales AC (2003). "Polymyxin-Resistant Acinetobacter spp. Isolates: What Is Next?". Emerg Infect Dis. 9 (8): 1025–7. doi:10.3201/eid0908.030052. PMC 3020604. PMID 12971377.
- Towner K J (2008). "Molecular Basis of Antibiotic Resistance in Acinetobacter spp.". Acinetobacter Molecular Biology. Caister Academic Press. ISBN 978-0-306-43902-5. Archived from the original on 2012-02-07.
- Benifla M, Zucker G, Cohen A, Alkan M (2004). "Successful treatment of Acinetobacter meningitis with intrathecal polymyxin". Journal of Antimicrobial Chemotherapy. 54 (1): 290–3. doi:10.1093/jac/dkh289. PMID 15190037.
- Yagmur R, Esen F (2006). "Intrathecal colistin for treatment of Pseudomonas aeruginosa ventriculitis: report of a case with successful outcome". Critical Care. 10 (6): 428. doi:10.1186/cc5088. PMC 1794456. PMID 17214907.
- Motaouakkil S, Charra B, Hachimi A, Nejmi H, Benslama A, Elmdaghri N, Belabbes H, Benbachir M (2006). "Colistin and rifampicin in the treatment of nosocomial infections from multiresistant Acinetobacter baumannii". Journal of Infection. 53 (4): 274–8. doi:10.1016/j.jinf.2005.11.019. PMID 16442632.
- Karakitsos D, Paramythiotou E, Samonis G, Karabinis A (2006). "Is intraventricular colistin an effective and safe treatment for post-surgical ventriculitis in the intensive care unit?". Acta Anaesthesiol. Scand. 50 (10): 1309–10. doi:10.1111/j.1399-6576.2006.01126.x. PMID 17067336.
- Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL (2006). "Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections". Lancet Infect Dis. 6 (9): 589–601. doi:10.1016/s1473-3099(06)70580-1. PMID 16931410.
- "Colomycin Injection". Summary of Product Characteristics. electronic Medicines Compendium (eMC). 18 May 2016. Archived from the original on 16 July 2017. Retrieved 3 June 2017.
- "COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS: COLISTIN: SUMMARY REPORT (2)" (PDF). European Medicines Agency. January 2002. Archived from the original (PDF) on 18 July 2006. NB. Colistin base has an assigned potency of 30 000 IU/mg
- Ahmed N, Wahlgren NG (2003). "In vitro interaction of colistin and rifampin on multidrug-resistant Pseudomonas aeruginosa". J Chemother. 15 (4): 235–8. doi:10.1159/000069498. PMID 12686786.
- Hogg GM, Barr JG, Webb CH (1998). "In-vitro activity of the combination of colistin and rifampicin against multidrug-resistant strains of Acinetobacter baumannii". J Antimicrob Chemother. 41 (4): 494–5. doi:10.1093/jac/41.4.494. PMID 9598783.
- Petrosillo N, Chinello P, Proietti MF, Cecchini L, Masala M, Franchi C, Venditti M, Esposito S, Nicastri E (2005). "Combined colistin and rifampicin therapy for carbapenem-resistant Acinetobacter baumannii infections: clinical outcome and adverse events". Clin Microbiol Infect. 11 (8): 682–3. doi:10.1111/j.1469-0691.2005.01198.x. PMID 16008625.
- MacGowan AP, Rynn C, Wootton M, Bowker KE, Holt HA, Reeves DS (1999). "In vitro assessment of colistin's antipseudomonal antimicrobial interactions with other antibiotics". Clin Microbiol Infect. 5 (1): 32–36. doi:10.1111/j.1469-0691.1999.tb00095.x. PMID 11856210.
- "Promixin 1 million International Units (IU) Powder for Nebuliser Solution". Patient Information Leafle. electronic Medicines Compendium (eMC). 12 January 2016. Archived from the original on 16 July 2017.
- Bruguera-Avila N, Marin A, Garcia-Olive I, Radua J, Prat C, Gil M, Ruiz-Manzano J (2017). "Effectiveness of treatment with nebulized colistin in patients with COPD". International Journal of Chronic Obstructive Pulmonary Disease. 12: 2909–2915. doi:10.2147/COPD.S138428. PMC 5634377. PMID 29042767.
- Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu LF, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu JH, Shen J (2016). "Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study". Lancet Infect Dis. 16 (2): 161–8. doi:10.1016/S1473-3099(15)00424-7. PMID 26603172.
- Zhang, Sarah. "Resistance to the Antibiotic of Last Resort Is Silently Spreading". The Atlantic. Archived from the original on 2017-01-13. Retrieved 2017-01-12.
- Maryn McKenna (2015-12-03). "Apocalypse Pig Redux: Last-Resort Resistance in Europe". Phenomena. Archived from the original on 28 May 2016. Retrieved 28 May 2016.
- "First discovery in United States of colistin resistance in a human E. coli infection". www.sciencedaily.com. Archived from the original on 2016-05-27. Retrieved 2016-05-27.
- "Emergence of Pan drug resistance amongst gram negative bacteria! The First case series from India". December 2014.
- "New worry: Resistance to 'last antibiotic' surfaces in India". 28 December 2014. Archived from the original on 31 December 2014.
- Bialvaei AZ, Samadi Kafil H (19 March 2015). "Colistin, mechanisms and prevalence of resistance". Curr Med Res Opin. 31 (4): 707–21. doi:10.1185/03007995.2015.1018989. PMID 25697677.
- "Discovery of first mcr-1 gene in E. coli bacteria found in a human in United States". cdc.gov. U.S. Department of Health and Human Services. 31 May 2016. Archived from the original on 2016-07-11. Retrieved 6 July 2016.
- Napier BA, Burd EM, Satola SW, Cagle SM, Ray SM, McGann P, Pohl J, Lesho EP, Weiss DS (21 May 2013). "Clinical Use of Colistin Induces Cross-Resistance to Host Antimicrobials in Acinetobacter baumannii". mBio. 4 (3): e00021–13–e00021–13. doi:10.1128/mBio.00021-13. PMC 3663567. PMID 23695834.
- "Common 'Superbug' Found to Disguise Resistance to Potent Antibiotic". wsj.com. Wall Street Journal. 6 March 2018. Archived from the original on 2018-04-03. Retrieved 1 Nov 2018.
- El-Halfawy OM, Valvano MA (2015). "Antimicrobial Heteroresistance: an Emerging Field in Need of Clarity". Clinical Microbiology Reviews. 28 (1): 191–207. doi:10.1128/CMR.00058-14. PMC 4284305. PMID 25567227.
- Band VI, Satola SW, Burd EM, Farley MM, Jacob JT, Weiss DS (2018). "Carbapenem-Resistant Klebsiella pneumoniae Exhibiting Clinically Undetected Colistin Heteroresistance Leads to Treatment Failure in a Murine Model of Infection". mBio. 9 (2): e02448–17. doi:10.1128/mBio.02448-17. PMC 5844991. PMID 29511071.
- Markou N, Apostolakos H, Koumoudiou C, Athanasiou M, Koutsoukou A, Alamanos I, Gregorakos L (2003). "Intravenous colistin in the treatment of sepsis from multiresistant Gram-negative bacilli in critically ill patients". Crit Care. 7 (5): R78–83. doi:10.1186/cc2358. PMC 270720. PMID 12974973.
- Wolinsky E, Hines JD (1962). "Neurotoxic and nephrotoxic effects of colistin in patients with renal disease". N Engl J Med. 266 (15): 759–68. doi:10.1056/NEJM196204122661505. PMID 14008070.
- Koch-Weser J, Sidel VW, Federman EB, Kanarek P, Finer DC, Eaton AE (1970). "Adverse effects of sodium colistimethate. Manifestations and specific reaction rates during 317 courses of therapy". Annals of Internal Medicine. 72 (6): 857–68. doi:10.7326/0003-4819-72-6-857. PMID 5448745.
- Ledson MJ, Gallagher MJ, Cowperthwaite C, Convery RP, Walshaw MJ (1998). "Four years' experience of intravenous colomycin in an adult cystic fibrosis unit". Eur Respir J. 12 (3): 592–4. doi:10.1183/09031936.98.12030592. PMID 9762785.
- Li J, Nation RL, Milne RW, Turnidge JD, Coulthard K (2005). "Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria". Int J Antimicrob Agents. 25 (1): 11–25. doi:10.1016/j.ijantimicag.2004.10.001. PMID 15620821.
- Beringer P (2001). "The clinical use of colistin in patients with cystic fibrosis". Curr Opin Pulm Med. 7 (6): 434–40. doi:10.1097/00063198-200111000-00013. PMID 11706322.
- Conway SP, Etherington C, Munday J, Goldman MH, Strong JJ, Wootton M (2000). "Safety and tolerability of bolus intravenous colistin in acute respiratory exacerbation in adults with cystic fibrosis". Annals of Pharmacotherapy. 34 (11): 1238–42. doi:10.1345/aph.19370. PMID 11098334.
- Littlewood JM, Koch C, Lambert PA, Høiby N, Elborn JS, Conway SP, Dinwiddie R, Duncan-Skingle F (2000). "A ten year review of Colomycin". Respir. Med. 94 (7): 632–40. doi:10.1053/rmed.2000.0834. PMID 10926333.
- Stein A, Raoult D (2002). "Colistin: an antimicrobial for the 21st century?". Clin Infect Dis. 35 (7): 901–2. doi:10.1086/342570. PMID 12228836.
- Giacobbe, Daniele Roberto; di Masi, Alessandra; Leboffe, Loris; Del Bono, Valerio; Rossi, Marianna; Cappiello, Dario; Coppo, Erika; Marchese, Anna; Casulli, Annarita; Signori, Alessio; Novelli, Andrea (December 2018). "Hypoalbuminemia as a predictor of acute kidney injury during colistin treatment". Scientific Reports. 8 (1): 11968. Bibcode:2018NatSR...811968G. doi:10.1038/s41598-018-30361-5. ISSN 2045-2322. PMC 6086859. PMID 30097635.
- Maddison J, Dodd M, Webb AK (1994). "Nebulized colistin causes chest tightness in adults with cystic fibrosis". Respir. Med. 88 (2): 145–7. doi:10.1016/0954-6111(94)90028-0. PMID 8146414.
- Kamin W, Schwabe A, Krämer I (2006). "Inhalation solutions: which one are allowed to be mixed? Physico-chemical compatibility of drug solutions in nebulizers". J. Cyst. Fibros. 5 (4): 205–213. doi:10.1016/j.jcf.2006.03.007. PMID 16678502.
- Domínguez-Ortega J, Manteiga E, Abad-Schilling C, Juretzcke MA, Sánchez-Rubio J, Kindelan C (2007). "Induced tolerance to nebulized colistin after severe reaction to the drug". J Investig Allergol Clin Immunol. 17 (1): 59–61. PMID 17323867.
- Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K (2004). "Pharmacokinetics of colistin methanesulphonate and colistin in rats following an intravenous dose of colistin methanesulphonate". J Antimicrob Chemother. 53 (5): 837–40. doi:10.1093/jac/dkh167. PMID 15044428.
- Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K (2003). "Use of High-Performance Liquid Chromatography To Study the Pharmacokinetics of Colistin Sulfate in Rats following Intravenous Administration". Antimicrob Agents Chemother. 47 (5): 1766–70. doi:10.1128/AAC.47.5.1766-1770.2003. PMC 153303. PMID 12709357.
- Y Koyama, A Kurosasa, A Tsuchiya, K Takakuta (1950). "A new antibiotic 'colistin' produced by spore-forming soil bacteria". J Antibiot (Tokyo). 3.CS1 maint: uses authors parameter (link)
- "Colistin: An overview". www.uptodate.com. Archived from the original on 2016-05-31. Retrieved 2016-06-06.
- Falagas M, Kasiakou S (2005). "Colistin: The Revival of Polymyxins for the Management of Multidrug-Resistant Gram-Negative Bacterial Infections". Rev Anti Infect Agen. 40. PMID 15825037.CS1 maint: uses authors parameter (link)
- Dewick, Paul M, Medicinal Natural Products, Third Edition, John Wiley & Sons, 2009
Further reading
- Reardon, Sara (21 December 2015). "Spread of antibiotic-resistance gene does not spell bacterial apocalypse — yet". Trend Watch. Nature. doi:10.1038/nature.2015.19037.
External links
- Drug information from the NIH about colistimethate sodium
- Drug information from the NIH about colistin sulfate
- "Colistin topics page (bibliography)". Science.gov.
- "Protocol for PCR detection of the gene mcr-1 gene" (PDF). National Food Institute. Technical University of Denmark. Archived from the original (PDF) on 2016-06-15. Retrieved 2016-05-27.