Cerebral edema
Cerebral edema is excess accumulation of fluid (edema) in the intracellular or extracellular spaces of the brain.[1] This typically causes impaired nerve function, increased pressure within the skull, and can eventually lead to direct compression of brain tissue and blood vessels.[1] Symptoms vary based on the location and extent of edema and generally include headaches, nausea, vomiting, drowsiness, visual disturbances, dizziness, and in severe cases, coma and death.[1]
| Cerebral Edema | |
|---|---|
| Other names | Brain Edema, [1] Cerebral oedema [2] |
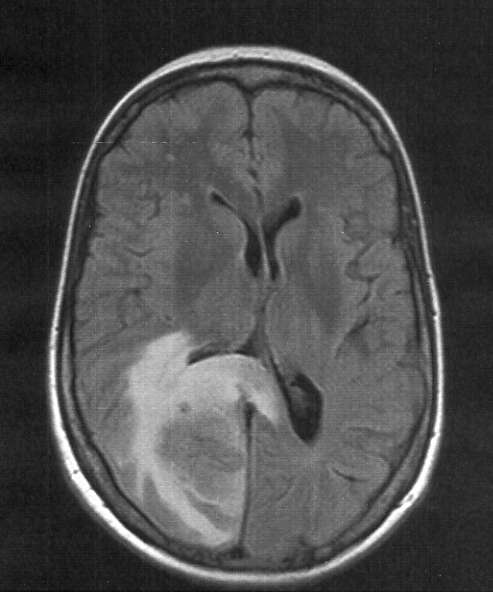 | |
| Skull MRI (T2 flair) of a brain metastasis with accompanying edema | |
| Specialty | Neurology |
| Symptoms | Headache, nausea, vomiting, decreased consciousness |
| Differential diagnosis | ischemic stroke, subdural hematoma, epidural hematoma, intracerebral hematoma, intraventricular hemorrhage, subarachnoid hemorrhage, hydrocephalus, traumatic brain injury, brain abscess, brain tumor, hyponatremia, hepatic encephalopathy |
Cerebral edema is commonly seen in a variety of brain injuries including ischemic stroke, subarachnoid hemorrhage, traumatic brain injury, subdural, epidural, or intracerebral hematoma, hydrocephalus, brain cancer, brain infections, low blood sodium levels, high altitude, and acute liver failure.[1][3][4][5][6] Diagnosis is based on symptoms and physical examination findings and confirmed by serial neuroimaging (computed tomography scans and magnetic resonance imaging).[3]
The treatment of cerebral edema depends on the cause and includes monitoring of the person's airway and intracranial pressure, proper positioning, controlled hyperventilation, medications, fluid management, steroids.[3][7][8] Extensive cerebral edema can also be treated surgically with a decompressive craniectomy.[7] Cerebral edema is a major cause of brain damage and contributes significantly to the mortality of ischemic strokes and traumatic brain injuries.[4][9]
As cerebral edema is present with many common cerebral pathologies, the epidemiology of the disease is not easily defined.[1] The incidence of this disorder should be considered in terms of its potential causes and is present in most cases of traumatic brain injury, central nervous system tumors, brain ischemia, and intracerebral hemorrhage.[1] For example, malignant brain edema was present in roughly 31% of people with ischemic strokes within 30 days after onset.[10]
Signs and symptoms
The extent and severity of the symptoms of cerebral edema depend on the exact etiology but are generally related to an acute increase of the pressure within the skull.[1] As the skull is a fixed and inelastic space, the accumulation of cerebral edema can displace and compress vital brain tissue, cerebral spinal fluid, and blood vessels, according to the Monroe-Kellie doctrine.[8]
Increased intracranial pressure (ICP) is a life-threatening surgical emergency marked by symptoms of headache, nausea, vomiting, decreased consciousness.[1] Symptoms are frequently accompanied by visual disturbances such as gaze paresis, reduced vision, and dizziness.[1] Increased pressures within the skull can cause a compensatory elevation of blood pressure to maintain cerebral blood flow, which, when associated with irregular breathing and a decreased heart rate, is called the Cushing reflex.[1] The Cushing reflex often indicates compression of the brain on brain tissue and blood vessels, leading to decreased blood flow to the brain and eventually death.[1]
Causes
Cerebral edema is frequently encountered in acute brain injuries from a variety of origins, including but not limited to: [7]
- Traumatic Brain Injury [8]
- Stroke [1]
- Tumors [1]
- Infections (such as a brain abscess or meningitis) [3][11]
- Hepatic Encephalopathy [5]
- Posterior Reversible Encephalopathy Syndrome [12]
- Radiation-induced brain edema [13]
- Post-surgical changes [14][15]
- High-Altitude Cerebral Edema [6]
- Amyloid-related imaging abnormalities associated with edema (ARIA-E) [16]
- Hyponatremia [17]
Risk Factors
Cerebral edema is a present with many common cerebral pathologies and risk factors for development of cerebral edema will depend on the cause.[1] The following were reliable predictors for development of early cerebral edema in ischemic strokes. [9][10]
- Younger age
- Higher severity of symptoms on the National Institutes of Health Stroke Scale
- Signs of current ischemia on clinical exam
- Decreased level of consciousness
- Hyper dense artery sign and larger affected area on CT imaging
- Higher blood glucose
Classification
Cerebral edema has been traditional classified into two major sub-types: cytotoxic and vasogenic cerebral edema.[1] This simple classification helps guide medical decision making and treatment of patients affected with cerebral edema.[3] There are, however, several more differentiated types including but not limited to interstitial, osmotic, hydrostatic, and high altitude associated edema.[1][3][7] Within one affected person, may individual sub-types can be present simultaneously.[18]
The following individual sub-types have been identified:
Cytotoxic
In general, cytotoxic edema is linked to cell death in the brain through excessive cellular swelling.[1] During cerebral ischemia for example, the blood–brain barrier remains intact but decreased blood flow and glucose supply leads to a disruption in cellular metabolism and creation of energy sources, such as adenosine triphosphate (ATP).[1] Exhaustion of energy sources impairs functioning of the sodium and potassium pump in the cell membrane, leading to cellular retention of sodium ions.[1] Accumulation of sodium in the cell causes a rapid uptake of water through osmosis, with subsequent swelling of the cells.[19] The ultimate consequence of cytotoxic edema is the oncotic death of neurons.[1] The swelling of the individual cells of the brain is the main distinguishing characteristic of cytotoxic edema, as opposed to vasogenic edema, wherein the influx of fluid is typically seen in the interstitial space rather than within the cells themselves.[20] Researchers have proposed that "cellular edema" may be more preferable to the term "cytotoxic edema" given the distinct swelling and lack of consistent "toxic" substance involved.[18]
There are several clinical conditions in which cytotoxic edema is present:
- Commonly caused by traumatic brain injuries, intracerebral hemorrhage, and the early phase of ischemic stroke.[1]
- Also seen in acute liver failure where toxic waste, most notably ammonia, accumulates in the blood stream and crosses the blood-brain barrier.[5] Hyperammonemia in central nervous system (CNS) cells causes oxidative stress and mitochrondrial dysfunction, leading to astrocytic cell swelling.[1] Additionally, ammonia is converted to glutamine in CNS cells which acts as an osmolyte and draws further water into the cell through osmosis.[5] Cerebral edema occurs most commonly in conjunction with a rapid rise in ammonia levels.[5]
- Toxic exposures to methionine sulfoxime, cuprizone, isoniazid, triethyl tin, hexachlorophene, and hydrogen cyanide have been associated with cytotoxic edema and swelling of astrocytic cells.[21]
- Hypoxia, anoxia can lead to cytotoxic edema through several mechanisms[18]
Vasogenic
Extracellular brain edema, or vasogenic edema, is caused by an increase in the permeability of the blood-brain barrier.[18] The blood-brain barrier consists of astrocytes and pericytes joined together with adhesion proteins producing tight junctions.[1] Return of blood flow to theses cells after an ischemic stroke can cause excitotoxicity and oxidative stress leading to dysfunction of the endothelial cells and disruption of the blood-brain barrier.[1] The breakdown of the tight endothelial junctions that make up the blood–brain barrier causes extravasation of fluid, ions, and plasma proteins, such as albumin, into the brain parenchyma.[18] Accumulation of extracellular fluid increases brain volume and then intracranial pressure causing the symptoms of cerebral edema.[1]
There are several clinical conditions in which vasogenic edema is present:
- CNS tumors, like glioblastoma and meningioma[1][21]
- Infections like meningitis, abscess, and encephalitis[1][21]
- Inflammatory central nervous system disease such as multiple sclerosis[1][22]
- Brain hemorrhage[21]
- Traumatic brain injuries can lead to increased intracranial pressure, local damage, reduced cerebral blood flow, and focal ischemia secondary to vasogenic edema.[4]
- Late stage of ischemic stroke after rapid recovery from cytotoxic edema[21]
- Hypertensive encephalopathy[21]
- Radiation injury[23]
Ionic (Osmotic)
In ionic edema, the solute concentration (osmolality) of the brain exceeds that of the plasma and the abnormal pressure gradient leads to accumulation of water intake into the brain parenchyma through the process of osmosis.[1] The blood-brain barrier is intact and maintains the osmotic gradient.[21]
The solute concentration of the blood plasma can be diluted by several mechanisms:
- Improper administration of intravenous fluids, isotonic or hypotonic.[21]
- Excessive water intake, syndrome of inappropriate antiduretic hormone (SIADH).[21]
- Rapid reduction of blood glucose in diabetic ketoacidosis or hyperosmolar hyperglycemic state.[18][21]
- Hemodialysis has been associated with ionic edema and cellular swelling.[18]
- Cerebral edema is a potentially life threatening complication of severely decreased sodium ion concentration in the blood (hyponatremia).[17]
Ionic brain edema can also occur around the sites of brain hemorrhages, infarcts, or contusions due to a local plasma osmolality pressure gradient when compared to the high osmolality in the affected tissue.[21]
Interstitial (Hydrocephalic)
Interstitial edema can be best characterized by in noncomunnicating hydrocephalus where there is an obstruction to the outflow of cerebrospinal fluid within the ventricular system.[1][21] The obstruction creates a rise the in intraventricular pressure and causes CSF to flow through the wall of the ventricles into the extracellular fluid within brain.[21] The fluid has roughly the same composition of CSF.[21]
Other causes of interstitial edema include but are not limited to communicating hydrocephalus, and normal pressure hydrocephalus.[18]
Hydrostatic
Hydrostatic extracellular brain edema is typically caused by severe arterial hypertension.[18] A difference in the hydrostatic pressure within the arterial system relative to the endothelial cells allows ultrafiltration of water, ions, and low molecular weight substances (such as glucose, small amino acids) into the brain parenchyma.[18] The blood-brain barrier is intact usually and the extent of the edema depends on the arterial pressure.[18] The regulatory processes of the brain circulation can function up to systolic arterial pressures of 150 mm Hg and will have impaired function at blood pressures higher.[18]
Combined types of cerebral edema
Cytotoxic, ionic, and vasogenic edema exist on a continuum.[24] The mechanism of the cause of cerebral edema can often overlap between these types.[24] In most instances, cytotoxic and vasogenic edema occur together.[25] When the two edema types evolve simultaneously, the damage of one type reaches a limit and will bring about the other type of injury.[25] For example, when cytotoxic edema occurs in the endothelial cells of the blood-brain barrier, oncotic cell death contributes to loss of integrity of the blood-brain barrier and promotes the progression to vasogenic edema.[24] When brain edema types are combined, there is typically a predominate form and the edema type and context of the cause must be determined in order to start appropriate medical or surgical therapy.[18] The use of specific MRI techniques has allowed for some differentiation between the mechanisms. [26]
Sub-types
High Altitude Cerebral Edema
If not properly acclimatized to high altitude, a person may be negatively affected by the lower oxygen concentration available.[27] These hypoxia-related illnesses include acute mountain sickness (AMS), high-altitude pulmonary edema, and high-altitude cerebral edema (HACE).[27] High altitude cerebral edema is a severe and sometimes fatal form of altitude sickness that results from capillary fluid leakage due to the effects of hypoxia on the mitochondria-rich endothelial cells of the blood–brain barrier.[28] The edema can be characterized by vasogenic cerebral edema with symptoms of impaired consciousness and truncal ataxia.[27]
Altitude-related illnesses can be prevented most effectively with slow ascent to high altitudes, an average ascent of 300 to 500 meters per day is recommended. Pharmacological prophylaxis with acetazoloamide or corticosteroids can be used in non pre-acclimatized individuals.[27] If symptoms of high-altitude cerebral edema do not resolve or worsen, immediate descent is necessary, and symptoms can be improved with administration of dexamethasone.[27]
Amyloid-Related Imaging Abnormalities - Edema
Amyloid-related imaging abnormalities (ARIA) are abnormal differences seen in neuroimaging of Alzheimer's disease patients given targeted amyloid-modifying therapies.[29] Human monoclonal antibodies such as aducanumab, solanezumab, and bapineuzumab have been associated with these neuroimaging changes and additionally, cerebral edema.[16][29] These therapies are associated with dysfunction of the tight endothelial junctions of the blood-brain barrier, leading to vasogenic edema as described above. In addition to edema, these therapies are associated with microhemorrhages in the brain known as ARIA-H.[30] Familiarity with ARIA can aid radiologists and clinicians in determining optimal management for those affected.[16]
Posterior Reversible Encephalopathy Syndrome
Posterior reversible encephalopathy syndrome is a rare clinical disease characterized by cerebral edema.[12] The exact pathophysiology, or cause, of the syndrome is still debated but is hypothesized to be related to the disruption of the blood-brain barrier.[12] The syndrome features acute neurological symptoms and reversible subcortical vasogenic edema predominantly involving the parieto-occipital areas on MR imaging.[31] PRES in general has a benign course, but PRES-related intracranial hemorrhage has been associated with a poor prognosis.[32]
Idiopathic delayed-onset edema
Deep brain stimulation (DBS) is effective treatment for several neurological and psychiatric disorders, most notably Parkinson's disease.[33] DBS is not without risks and although rare, idiopathic delayed-onset edema (IDE) surrounding the DBS leads have been reported.[14] Symptoms can be mild and nonspecific, including reduction of the stimulation effect, and can be confused for other causes of edema.[14] Thus, imaging is recommended to rule out other causes.[14] The condition is generally self-limiting and the exact mechanism of the cause is unexplained.[14] Early identification can help persons affected avoid unnecessary surgical procedures or antibiotic treatments.[14]
Massive Brain Swelling after Cranioplasty
Decompressive craniectomy is frequently performed in cases of resistant intracranial hypertension secondary to several neurological conditions and is commonly followed by cranioplasty.[15] Complications, such as infection and hematomas after cranioplasty occur in roughly about a third of cases.[15] Massive brain swelling after cranioplasty (MSBC) is a rare and potentially fatal complication of an uneventful cranioplasty that has recently been elucidated.[15] Preoperative sinking skin flap (SSF) and intracranial hypotension were factors associated with the development of MSBC after cranioplasty.[15][34] Data suggests that pathologic changes are triggered immediately following the procedure, especially an acute increase in intracranial pressure.[15]
Radiation-Induced Brain Edema
With the rise of sophisticated treatment modalities such as gamma knife, cyber knife, and intensity modulated radiotherapy, a large number of individuals with brain tumors are treated with radiosurgery and radiotherapy.[13] Radiation-induced brain edema (RIBE) is a potentially life threatening complication of brain tissue radiation and is characterized radiation necrosis, endothelial cell dysfunction, increased capillary permeability, and breakdown of the blood brain barrier.[13] Symptoms include headache, seizure, psychomotor slowing, irritability, and focal neurological deficits.[13] Options for management of RIBE are limited and include corticosteroids, anti platelet drugs, anticoagulants, hyperbaric oxygen therapy, multivitamins, and bevacizumab.[13]
Brain tumor-associated cerebral edema
Brain tumor associated edema is a significant cause of morbidity and mortality in patients with brain tumors and characterized by a disruption of the blood brain barrier and vasogenic edema.[35] The exact mechanism is unclear but hypothesized that cancerous glial cells (glioma) of the brain can increase secretion of vascular endothelial growth factor (VEGF), which weakens the junctions of the blood–brain barrier.[36] Historically, corticosteroids such as dexamethasone were used to reduce brain tumor-associated vascular permeability through poorly understood mechanisms and was associated with systemic side effects.[36] Agents that target the VEGF signaling pathways, such as cediranib, have been promising in prolonging survival in rat models but associated with local and systemic side effects.[35]
Diagnosis
Cerebral edema is commonly present in a variety of neurological injuries.[1][3] Thus, determining a definitive contribution of cerebral edema to the neurological status of an affected person can be challenging.[3] Close bedside monitoring of a person's level of consciousness and awareness of any new or worsening focal neurological deficits is imperative but demanding, frequently requiring admission into the intensive care unit (ICU).[3]
Cerebral edema with sustained increased intracranial hypertension and brain herniation can signify impending catastrophic neurological events which require immediate recognition and treatment to prevent injury and even death.[1][9][10][37] Therefore, diagnosis of cerebral edema earlier with rapid intervention can improve clinical outcomes and can mortality, or risk of death.[37]
Diagnosis of cerebral edema relies on the following:
Imaging
Serial neuroimaging (CT scans and magnetic resonance imaging) can be useful in diagnosing or excluding intracranial hemorrhage, large masses, acute hydrocephalus, or brain herniation as well as providing information on the type of edema present and the extent of affected area.[1][3] CT scan is the imaging modality of choice as it is widely available, quick, and with minimal risks.[1] However, CT scan can be limited in determining the exact cause of cerebral edema in which cases, CT angiography (CTA), MRI, or digital subtraction angiography (DSA) may be necessary. MRI is particularly useful as it can differentiate between cytotoxic and vasogenic edema, guiding future treatment decisions.[1]
Intracranial pressure monitoring
Intracranial pressure and its management is a fundamental concept in traumatic brain injury (TBI).[38] The Brain Trauma Foundation guidelines recommend ICP monitoring in individuals with TBI that have decreased Glasgow-Coma Scale (GCS) scores, abnormal CT scans, or additional risk factors such as older age and elevated blood pressure.[3] However, no such guidelines exist for ICP monitoring in other brain injuries such as ischemic stroke, intracerebral hemorrhage, cerebral neoplasm.[3]
Clinical researches have recommended ICP and cerebral perfusion pressure (CPP) monitoring in any persons with cerebral injury who are at risk of elevated intracranial pressure based on clinical and neuroimaging features.[38] Early monitoring can be used to guide medical and surgical decision making and to detect potentially life-threatening brain herniation.[38] There was however, conflicting evidence on the threshold values of ICP that indicated the need for intervention.[38] Researches also recommend that medical decisions should be tailored to the specific diagnosis (e.g. subarachnoid hemorrhage, TBI, encephalitis) and that ICP elevation should be used in conjunction with clinical and neuroimaging and not as an isolated prognostic marker.[38]
Treatment
Treatment approaches can include osmotherapy using mannitol, diuretics to decrease fluid volume, corticosteroids to suppress the immune system, hypertonic saline, and surgical decompression to allow the brain tissue room to swell without compressive injury.[3][39]
Research
Many studies of the mechanical properties of brain edema were conducted in the 2010s, most of them based on finite element analysis (FEA), a widely used numerical method in solid mechanics. For example, Gao and Ang used the finite element method to study changes in intracranial pressure during craniotomy operations.[40] A second line of research on the condition looks at thermal conductivity, which is related to tissue water content.[41]
See also
- Amyloid-related imaging abnormalities
- Edema
References
- Leinonen, Ville; Vanninen, Ritva; Rauramaa, Tuomas (2018), "Raised intracranial pressure and brain edema", Handbook of Clinical Neurology, Elsevier, 145, pp. 25–37, doi:10.1016/b978-0-12-802395-2.00004-3, ISBN 978-0-12-802395-2, retrieved 2020-04-06
- 'Oedema' is the standard form defined in the Concise Oxford English Dictionary (2011), with the precision that the spelling in the United States is 'edema'.
- Raslan A, Bhardwaj A (2007). "Medical management of cerebral edema". Neurosurgical Focus. 22 (5): E12. doi:10.3171/foc.2007.22.5.13. PMID 17613230.
- Lahner, D.; Fritsch, G. (September 2017). "[Pathophysiology of intracranial injuries]". Der Unfallchirurg. 120 (9): 728–733. doi:10.1007/s00113-017-0388-0. ISSN 1433-044X. PMID 28812113.
- Wijdicks, Eelco F. M. (2016-10-27). "Hepatic Encephalopathy". The New England Journal of Medicine. 375 (17): 1660–1670. doi:10.1056/NEJMra1600561. ISSN 1533-4406. PMID 27783916.
- Dehnert, Christoph; Bärtsch, Peter (2017). "[Acute Mountain Sickness and High-Altitude Cerebral Edema]". Therapeutische Umschau. Revue Therapeutique. 74 (10): 535–541. doi:10.1024/0040-5930/a000954. ISSN 0040-5930. PMID 29690831.
- Adukauskiene, Dalia; Bivainyte, Asta; Radaviciūte, Edita (2007). "[Cerebral edema and its treatment]". Medicina (Kaunas, Lithuania). 43 (2): 170–176. ISSN 1648-9144. PMID 17329953.
- Jha, Ruchira M.; Kochanek, Patrick M. (November 7, 2018). "A Precision Medicine Approach to Cerebral Edema and Intracranial Hypertension after Severe Traumatic Brain Injury: Quo Vadis?". Current Neurology and Neuroscience Reports. 18 (12): 105. doi:10.1007/s11910-018-0912-9. ISSN 1534-6293. PMC 6589108. PMID 30406315.
- Thorén, Magnus; Azevedo, Elsa; Dawson, Jesse; Egido, Jose A.; Falcou, Anne; Ford, Gary A.; Holmin, Staffan; Mikulik, Robert; Ollikainen, Jyrki; Wahlgren, Nils; Ahmed, Niaz (September 2017). "Predictors for Cerebral Edema in Acute Ischemic Stroke Treated With Intravenous Thrombolysis". Stroke. 48 (9): 2464–2471. doi:10.1161/STROKEAHA.117.018223. ISSN 1524-4628. PMID 28775140.
- Wu, Simiao; Yuan, Ruozhen; Wang, Yanan; Wei, Chenchen; Zhang, Shihong; Yang, Xiaoyan; Wu, Bo; Liu, Ming (December 2018). "Early Prediction of Malignant Brain Edema After Ischemic Stroke". Stroke. 49 (12): 2918–2927. doi:10.1161/STROKEAHA.118.022001. ISSN 1524-4628. PMID 30571414.
- Simjian, Thomas; Muskens, Ivo S.; Lamba, Nayan; Yunusa, Ismaeel; Wong, Kristine; Veronneau, Raymond; Kronenburg, Annick; Brouwers, H. Bart; Smith, Timothy R.; Mekary, Rania A.; Broekman, Marike L. D. (July 2018). "Dexamethasone Administration and Mortality in Patients with Brain Abscess: A Systematic Review and Meta-Analysis". World Neurosurgery. 115: 257–263. doi:10.1016/j.wneu.2018.04.130. ISSN 1878-8769. PMID 29705232.
- Largeau, Bérenger; Boels, David; Victorri-Vigneau, Caroline; Cohen, Clara; Salmon Gandonnière, Charlotte; Ehrmann, Stephan (2019). "Posterior Reversible Encephalopathy Syndrome in Clinical Toxicology: A Systematic Review of Published Case Reports". Frontiers in Neurology. 10: 1420. doi:10.3389/fneur.2019.01420. ISSN 1664-2295. PMC 7029435. PMID 32116991.
- Tripathi, Manjul; Ahuja, Chirag K.; Mukherjee, Kanchan K.; Kumar, Narendra; Dhandapani, Sivashanmugam; Dutta, Pinaki; Kaur, Rupinder; Rekhapalli, Rajashekhar; Batish, Aman; Gurnani, Jenil; Kamboj, Parwinder (September 2019). "The Safety and Efficacy of Bevacizumab for Radiosurgery - Induced Steroid - Resistant Brain Edema; Not the Last Part in the Ship of Theseus". Neurology India. 67 (5): 1292–1302. doi:10.4103/0028-3886.271242. ISSN 1998-4022. PMID 31744962.
- de Cuba, Catherine M. K. E.; Albanese, Alberto; Antonini, Angelo; Cossu, Giovanni; Deuschl, Günther; Eleopra, Roberto; Galati, Alejandro; Hoffmann, Carel F. E.; Knudsen, Karina; Landi, Andrea; Lanotte, Michele Maria R. (November 2016). "Idiopathic delayed-onset edema surrounding deep brain stimulation leads: Insights from a case series and systematic literature review". Parkinsonism & Related Disorders. 32: 108–115. doi:10.1016/j.parkreldis.2016.09.007. ISSN 1873-5126. PMID 27622967.
- Robles, Luis A.; Cuevas-Solórzano, Abel (March 2018). "Massive Brain Swelling and Death After Cranioplasty: A Systematic Review". World Neurosurgery. 111: 99–108. doi:10.1016/j.wneu.2017.12.061. ISSN 1878-8769. PMID 29269069.
- Barakos, J.; Sperling, R.; Salloway, S.; Jack, C.; Gass, A.; Fiebach, J. B.; Tampieri, D.; Melançon, D.; Miaux, Y.; Rippon, G.; Black, R. (October 2013). "MR imaging features of amyloid-related imaging abnormalities". AJNR. American journal of neuroradiology. 34 (10): 1958–1965. doi:10.3174/ajnr.A3500. ISSN 1936-959X. PMID 23578674.
- Adrogué, H. J.; Madias, N. E. (2000-05-25). "Hyponatremia". The New England Journal of Medicine. 342 (21): 1581–1589. doi:10.1056/NEJM200005253422107. ISSN 0028-4793. PMID 10824078.
- Iencean, S. M. (July 2003). "Brain edema -- a new classification". Medical Hypotheses. 61 (1): 106–109. doi:10.1016/s0306-9877(03)00127-0. ISSN 0306-9877. PMID 12781651.
- Rosenberg, Gary (1999). "Ischemic Brain Edema". Progress in Cardiovascular Diseases. 42 (3): 209–16. doi:10.1016/s0033-0620(99)70003-4. PMID 10598921.
- Klatzo, Igor (1 January 1987). "Pathophysiological aspects of brain edema". Acta Neuropathologica. 72 (3): 236–239. doi:10.1007/BF00691095.
- Nag, Sukriti; Manias, Janet L.; Stewart, Duncan J. (August 2009). "Pathology and new players in the pathogenesis of brain edema". Acta Neuropathologica. 118 (2): 197–217. doi:10.1007/s00401-009-0541-0. ISSN 1432-0533. PMID 19404652.
- Argaw, Azeb Tadesse; Asp, Linnea; Zhang, Jingya; Navrazhina, Kristina; Pham, Trinh; Mariani, John N.; Mahase, Sean; Dutta, Dipankar J.; Seto, Jeremy; Kramer, Elisabeth G.; Ferrara, Napoleone (2012-07-02). "Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease". The Journal of Clinical Investigation. 122 (7): 2454–2468. doi:10.1172/JCI60842. ISSN 0021-9738. PMID 22653056.
- Milano, Michael T.; Sharma, Manju; Soltys, Scott G.; Sahgal, Arjun; Usuki, Kenneth Y.; Saenz, Jon-Michael; Grimm, Jimm; El Naqa, Issam (July 1, 2018). "Radiation-Induced Edema After Single-Fraction or Multifraction Stereotactic Radiosurgery for Meningioma: A Critical Review". International Journal of Radiation Oncology, Biology, Physics. 101 (2): 344–357. doi:10.1016/j.ijrobp.2018.03.026. ISSN 1879-355X. PMID 29726362.
- Jha, Ruchira M.; Kochanek, Patrick M. (November 7, 2018). "A Precision Medicine Approach to Cerebral Edema and Intracranial Hypertension after Severe Traumatic Brain Injury: Quo Vadis?". Current Neurology and Neuroscience Reports. 18 (12): 105. doi:10.1007/s11910-018-0912-9. ISSN 1534-6293. PMC 6589108. PMID 30406315.
- Iencean, S. M. (July 2003). "Brain edema -- a new classification". Medical Hypotheses. 61 (1): 106–109. doi:10.1016/s0306-9877(03)00127-0. ISSN 0306-9877. PMID 12781651.
- Barzó, P; Marmarou, A; Fatouros, P; Hayasaki, K; Corwin, F (December 1997). "Contribution of vasogenic and cellular edema to traumatic brain swelling measured by diffusion-weighted imaging". Journal of Neurosurgery. 87 (6): 900–7. doi:10.3171/jns.1997.87.6.0900. PMID 9384402.
- Dehnert, Christoph; Bärtsch, Peter (2018-04-25). "Akute Bergkrankheit und Höhenhirnödem". Therapeutische Umschau (in German). doi:10.1024/0040-5930/a000954. ISSN 0040-5930.
- Van Osta A, Moraine JJ, Mélot C, Mairbäurl H, Maggiorini M, Naeije R (2005). "Effects of high altitude exposure on cerebral hemodynamics in normal subjects". Stroke. 36 (3): 557–560. doi:10.1161/01.STR.0000155735.85888.13. PMID 15692117.
- Sperling, Reisa A.; Jack, Clifford R.; Black, Sandra E.; Frosch, Matthew P.; Greenberg, Steven M.; Hyman, Bradley T.; Scheltens, Philip; Carrillo, Maria C.; Thies, William; Bednar, Martin M.; Black, Ronald S. (July 2011). "Amyloid Related Imaging Abnormalities (ARIA) in Amyloid Modifying Therapeutic Trials: Recommendations from the Alzheimer's Association Research Roundtable Workgroup". Alzheimer's & dementia : the journal of the Alzheimer's Association. 7 (4): 367–385. doi:10.1016/j.jalz.2011.05.2351. ISSN 1552-5260. PMC 3693547. PMID 21784348.
- van Dyck, Christopher H. (February 15, 2018). "Anti-Amyloid-β Monoclonal Antibodies for Alzheimer's Disease: Pitfalls and Promise". Biological Psychiatry. 83 (4): 311–319. doi:10.1016/j.biopsych.2017.08.010. ISSN 1873-2402. PMC 5767539. PMID 28967385.
- González Quarante, Lain Hermes; Mena-Bernal, José Hinojosa; Martín, Beatriz Pascual; Ramírez Carrasco, Marta; Muñoz Casado, María Jesús; Martínez de Aragón, Ana; de las Heras, Rogelio Simón (May 2016). "Posterior reversible encephalopathy syndrome (PRES): a rare condition after resection of posterior fossa tumors: two new cases and review of the literature". Child's Nervous System: ChNS: Official Journal of the International Society for Pediatric Neurosurgery. 32 (5): 857–863. doi:10.1007/s00381-015-2954-5. ISSN 1433-0350. PMID 26584552.
- Yamagami, Keitaro; Maeda, Yoshihisa; Iihara, Koji (February 2020). "Variant Type of Posterior Reversible Encephalopathy Syndrome Associated with Deep Brain Hemorrhage: Case Report and Review of the Literature". World Neurosurgery. 134: 176–181. doi:10.1016/j.wneu.2019.10.196. ISSN 1878-8769. PMID 31712110.
- Kocabicak, Ersoy; Temel, Yasin; Höllig, Anke; Falkenburger, Björn; Tan, Sonny Kh (2015). "Current perspectives on deep brain stimulation for severe neurological and psychiatric disorders". Neuropsychiatric Disease and Treatment. 11: 1051–1066. doi:10.2147/NDT.S46583. ISSN 1176-6328. PMC 4399519. PMID 25914538.
- Khan, Noman Ahmed Jang; Ullah, Saad; Alkilani, Waseem; Zeb, Hassan; Tahir, Hassan; Suri, Joshan (2018). "Sinking Skin Flap Syndrome: Phenomenon of Neurological Deterioration after Decompressive Craniectomy". Case Reports in Medicine. Retrieved 2020-04-09.
- Ong, Qunya; Hochberg, Fred H.; Cima, Michael J. (2015-11-10). "Depot delivery of dexamethasone and cediranib for the treatment of brain tumor associated edema in an intracranial rat glioma model". Journal of Controlled Release: Official Journal of the Controlled Release Society. 217: 183–190. doi:10.1016/j.jconrel.2015.08.028. ISSN 1873-4995. PMID 26285064.
- Heiss JD, Papavassiliou E, Merrill MJ, Nieman L, Knightly JJ, Walbridge S, Edwards NA, Oldfield EH (1996). "Mechanism of dexamethasone suppression of brain tumor-associated vascular permeability in rats. Involvement of the glucocorticoid receptor and vascular permeability factor". Journal of Clinical Investigation. 98 (6): 1400–1408. doi:10.1172/JCI118927. PMC 507566. PMID 8823305.
- Tucker, Brian; Aston, Jill; Dines, Megan; Caraman, Elena; Yacyshyn, Marianne; McCarthy, Mary; Olson, James E. (July 2017). "Early Brain Edema is a Predictor of In-Hospital Mortality in Traumatic Brain Injury". The Journal of Emergency Medicine. 53 (1): 18–29. doi:10.1016/j.jemermed.2017.02.010. ISSN 0736-4679. PMID 28343797.
- Chesnut, Randall; Videtta, Walter; Vespa, Paul; Le Roux, Peter; Participants in the International Multidisciplinary Consensus Conference on Multimodality Monitoring (December 2014). "Intracranial pressure monitoring: fundamental considerations and rationale for monitoring". Neurocritical Care. 21 Suppl 2: S64–84. doi:10.1007/s12028-014-0048-y. ISSN 1556-0961. PMID 25208680.
- Mortazavi, Martin M.; Romeo, Andrew K.; Deep, Aman; Griessenauer, Christoph J.; Shoja, Mohammadali M.; Tubbs, R. Shane; Fisher, Winfield (2012). "Hypertonic saline for treating raised intracranial pressure: literature review with meta-analysis". Journal of Neurosurgery. 116 (1): 210–221. doi:10.3171/2011.7.jns102142.
- Gao CP, Ang BT (2008). "Biomechanical modeling of decompressive craniectomy in traumatic brain injury". Acta Neurochirurgica. 102 (supplement): 279–282. doi:10.1007/978-3-211-85578-2_52.
- Ko S.-B.; Choi H. Alex; Parikh G.; Schmidt J. Michael; Lee K.; Badjatia N.; Claassen J.; Connolly E. Sander; Mayer S. A. (2012). "Real time estimation of brain water content in comatose patients". Ann. Neurol. 72: 344–50. doi:10.1002/ana.23619. PMC 3464349. PMID 22915171.